Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In equimolar counter diffusion for every mole of one component diffusing in one direction there is , by definition, one mole of the other component
In equimolar counter diffusion for every mole of one component diffusing in one direction there is by definition, one mole of the other component diffusing in the opposite direction.
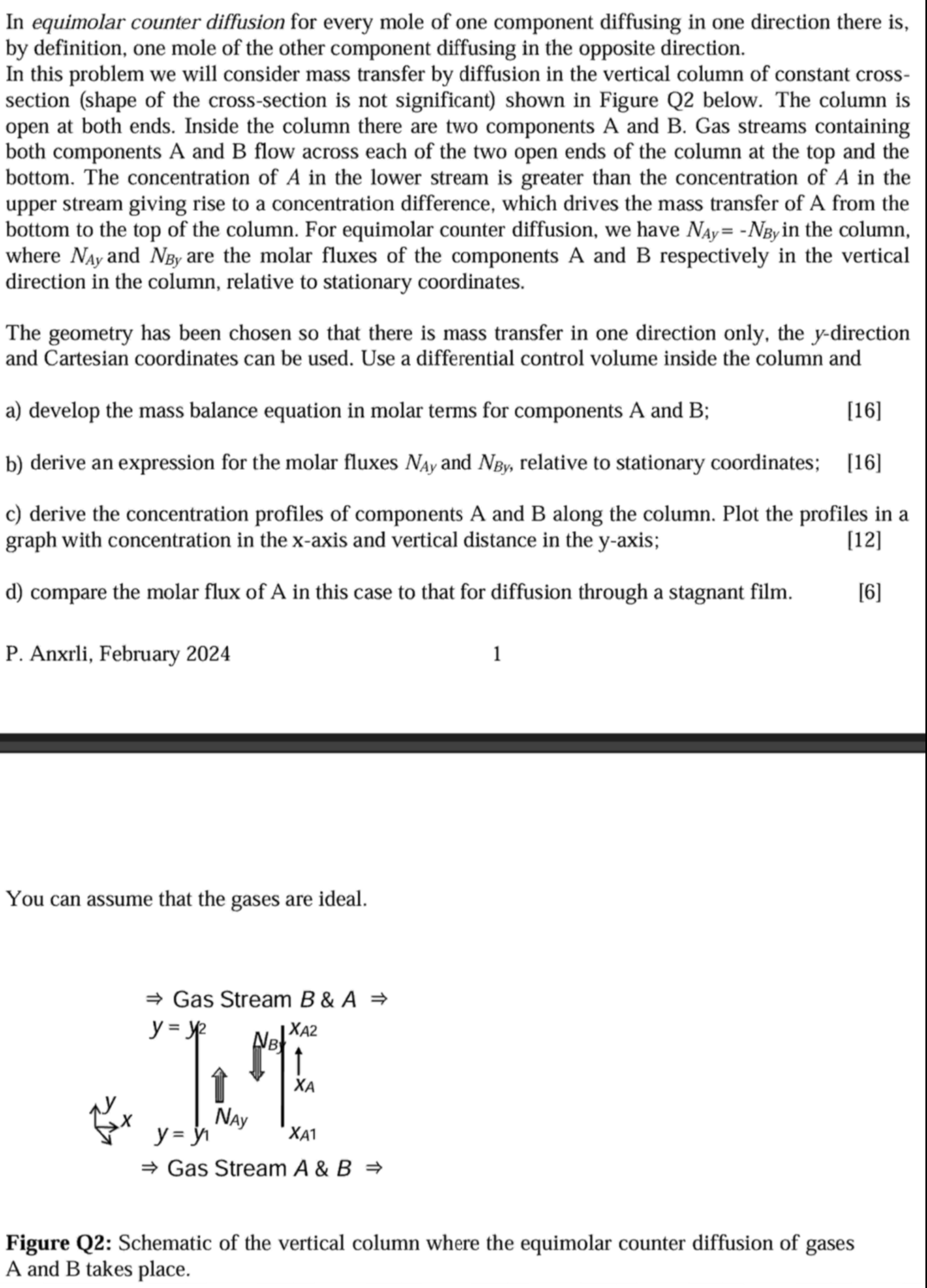
In this problem we will consider mass transfer by diffusion in the vertical column of constant cross section shape of the crosssection is not significant shown in Figure Q below. The column is open at both ends. Inside the column there are two components A and B Gas streams containing both components A and B flow across each of the two open ends of the column at the top and the bottom. The concentration of A in the lower stream is greater than the concentration of A in the upper stream giving rise to a concentration difference, which drives the mass transfer of A from the bottom to the top of the column. For equimolar counter diffusion, we have NAy NBy in the column, where NAy and NBy are the molar fluxes of the components A and B respectively in the vertical direction in the column, relative to stationary coordinates.
The geometry has been chosen so that there is mass transfer in one direction only, the ydirection and Cartesian coordinates can be used. Use a differential control volume inside the column and
a develop the mass balance equation in molar terms for components A and B;
b derive an expression for the molar fluxes NAy and NBy relative to stationary coordinates;
c derive the concentration profiles of components A and B along the column. Plot the profiles in a graph with concentration in the xaxis and vertical distance in the yaxis;
d compare the molar flux of A in this case to that for diffusion through a stagnant film.
You can assume that the gases are ideal.
Gas Stream B & A
y y
x y y NAy
NBy xA
xA xA
Gas Stream A & B In equimolar counter diffusion for every mole of one component diffusing in one direction there is
by definition, one mole of the other component diffusing in the opposite direction.
In this problem we will consider mass transfer by diffusion in the vertical column of constant cross
section shape of the crosssection is not significant shown in Figure Q below. The column is
open at both ends. Inside the column there are two components A and B Gas streams containing
both components A and B flow across each of the two open ends of the column at the top and the
bottom. The concentration of in the lower stream is greater than the concentration of in the
upper stream giving rise to a concentration difference, which drives the mass transfer of A from the
bottom to the top of the column. For equimolar counter diffusion, we have in the column,
where and are the molar fluxes of the components A and B respectively in the vertical
direction in the column, relative to stationary coordinates.
The geometry has been chosen so that there is mass transfer in one direction only, the direction
and Cartesian coordinates can be used. Use a differential control volume inside the column and
a develop the mass balance equation in molar terms for components A and ;
b derive an expression for the molar fluxes and relative to stationary coordinates;
c derive the concentration profiles of components A and along the column. Plot the profiles in a
graph with concentration in the axis and vertical distance in the axis;
d compare the molar flux of in this case to that for diffusion through a stagnant film.
You can assume that the gases are ideal.
Figure Q: Schematic of the vertical column where the equimolar counter diffusion of gases
A and B takes place.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


