Question
In f-element compounds like Nd3+, there are typically large energy splittings between J values, and absorption spectra show transitions between different J values. How
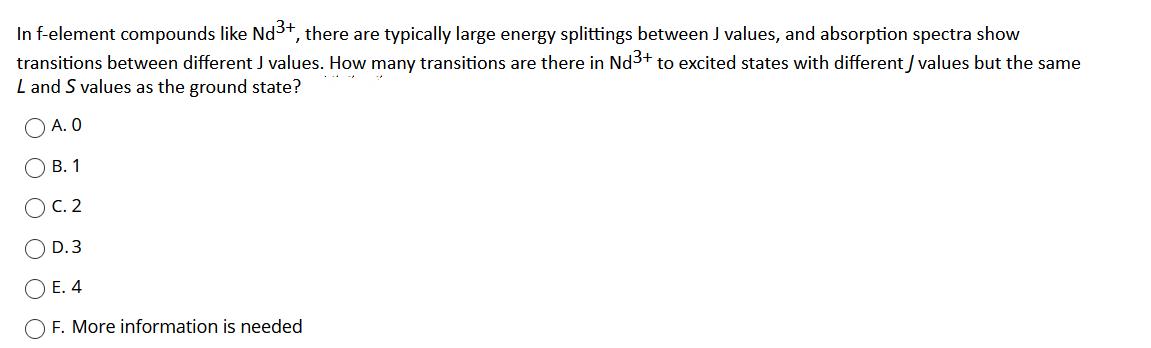
In f-element compounds like Nd3+, there are typically large energy splittings between J values, and absorption spectra show transitions between different J values. How many transitions are there in Nd3+ to excited states with different / values but the same L and S values as the ground state? A. 0 . 1 . 2 D. 3 E. 4 O F. More information is needed O O O O
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Here Nd has a 4f3 electronic configuration It has a ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
A First Course In Probability
Authors: Sheldon Ross
9th Edition
978-9332519077, 9332519072
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



