Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In Figure 1, a rubber vessel is filled with carbon dioxide gas at 298K and 65 bar. A 50- mm diameter circular tube rubber
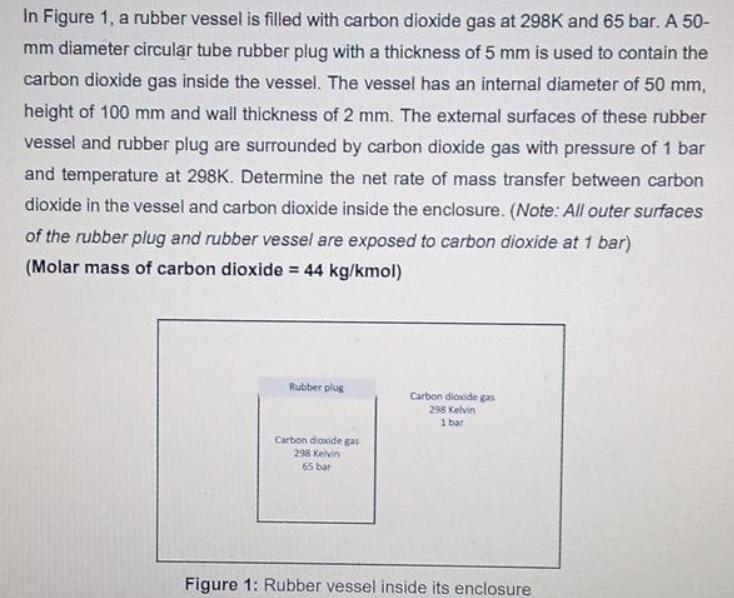
In Figure 1, a rubber vessel is filled with carbon dioxide gas at 298K and 65 bar. A 50- mm diameter circular tube rubber plug with a thickness of 5 mm is used to contain the carbon dioxide gas inside the vessel. The vessel has an internal diameter of 50 mm, height of 100 mm and wall thickness of 2 mm. The external surfaces of these rubber vessel and rubber plug are surrounded by carbon dioxide gas with pressure of 1 bar and temperature at 298K. Determine the net rate of mass transfer between carbon dioxide in the vessel and carbon dioxide inside the enclosure. (Note: All outer surfaces of the rubber plug and rubber vessel are exposed to carbon dioxide at 1 bar) (Molar mass of carbon dioxide = 44 kg/kmol) Rubber plug Carbon dioxide gas 298 Kelvin 65 bar Carbon dioxide gas 295 Kelvin 1 bar Figure 1: Rubber vessel inside its enclosure
Step by Step Solution
★★★★★
3.56 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION To determine the net rate of mass transfer between the carbon dioxide in the vessel and the carbon dioxide inside the enclosure we need to consider three main steps 1 Calculate the surface ar...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


