Answered step by step
Verified Expert Solution
Question
1 Approved Answer
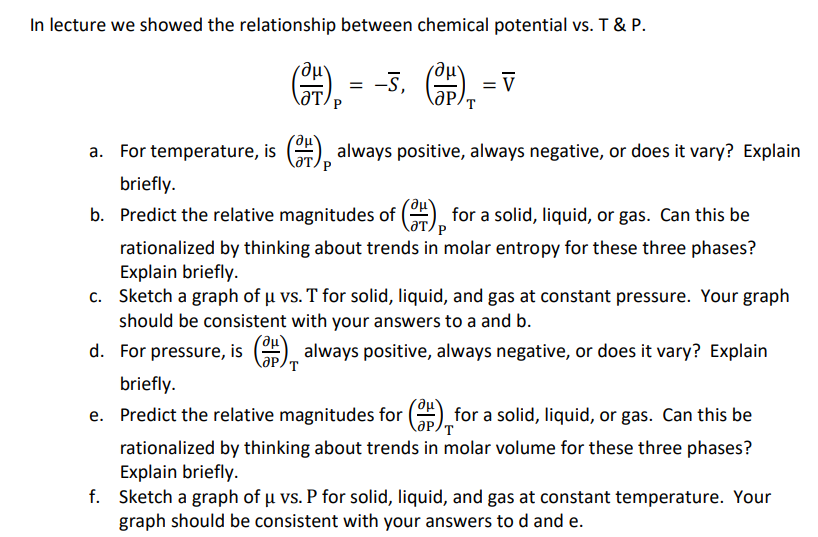
In lecture we showed the relationship between chemical potential vs . T & P . ( d e l d e l T ) P
In lecture we showed the relationship between chemical potential vs T & P
a For temperature, is always positive, always negative, or does it vary? Explain
briefly.
b Predict the relative magnitudes of for a solid, liquid, or gas. Can this be
rationalized by thinking about trends in molar entropy for these three phases?
Explain briefly.
c Sketch a graph of vs T for solid, liquid, and gas at constant pressure. Your graph
should be consistent with your answers to a and
d For pressure, is always positive, always negative, or does it vary? Explain
briefly.
e Predict the relative magnitudes for for a solid, liquid, or gas. Can this be
rationalized by thinking about trends in molar volume for these three phases?
Explain briefly.
f Sketch a graph of vs P for solid, liquid, and gas at constant temperature. Your
graph should be consistent with your answers to and
Please Make sure everything is correct because Im having wrong answers on chegg always and that is affecting my academic goals badly. Thank you so much for your help
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


