Question
A) A sample of an ideal gas undergo an isobaric process. Given that the pressure is 1 atm, and the volume of ideal gas
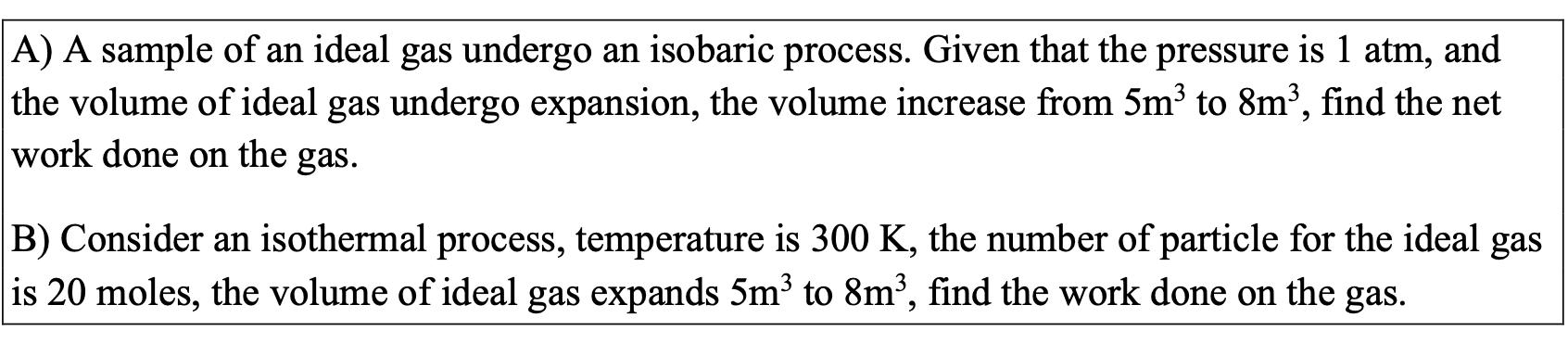
A) A sample of an ideal gas undergo an isobaric process. Given that the pressure is 1 atm, and the volume of ideal gas undergo expansion, the volume increase from 5m to 8m, find the net work done on the gas. B) Consider an isothermal process, temperature is 300 K, the number of particle for the ideal gas is 20 moles, the volume of ideal gas expands 5m to 8m, find the work done on the gas.
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
A In an isobaric process the pressure remains constant The work done on or by the gas can be calcula...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cornerstones of Financial and Managerial Accounting
Authors: Rich Jones, Mowen, Hansen, Heitger
1st Edition
9780538751292, 324787359, 538751290, 978-0324787351
Students also viewed these General Management questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



