Question
In Prof. Tokuyamas synthesis, the oxazolinone (A) was deprotonated to generate an enolate (B) with defined stereochemistry, which then participated in an aldol reaction with
In Prof. Tokuyama’s synthesis, the oxazolinone (A) was deprotonated to generate an enolate (B) with defined stereochemistry, which then participated in an aldol reaction with the chiral aldehyde (C) to give the product (D).
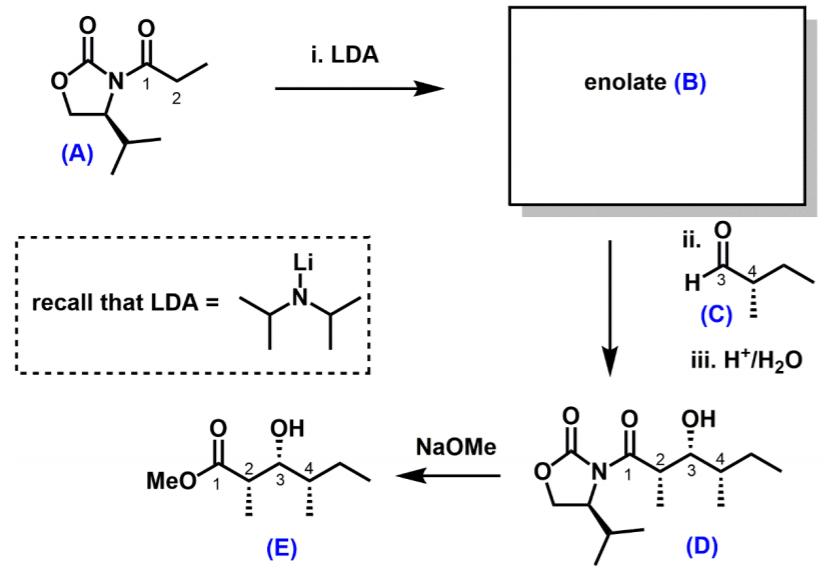
(a) Suggest a structure for enolate (B) (2 marks)
(b) Using cyclic deprotonation models, justify why the enolate (B) has a particular stereochemistry (E or Z). (8 marks)
(c) Using Newman projects, account for why compound (D) has the syn stereochemical relationship between the stereogenic centres labelled 3 and 4. (8 marks)
(d) When the auxiliary is cleaved, will the product compound (E) be a single enantiomer, racemic, or achiral? Justify your answer. (4 marks)
i. LDA N' enolate (B) (A) ii. Li 4 recall that LDA = H3 (C) iii. H*/H20 OH OH NaOMe MeO 1 4 3 (E) (D)
Step by Step Solution
3.38 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



