In Python

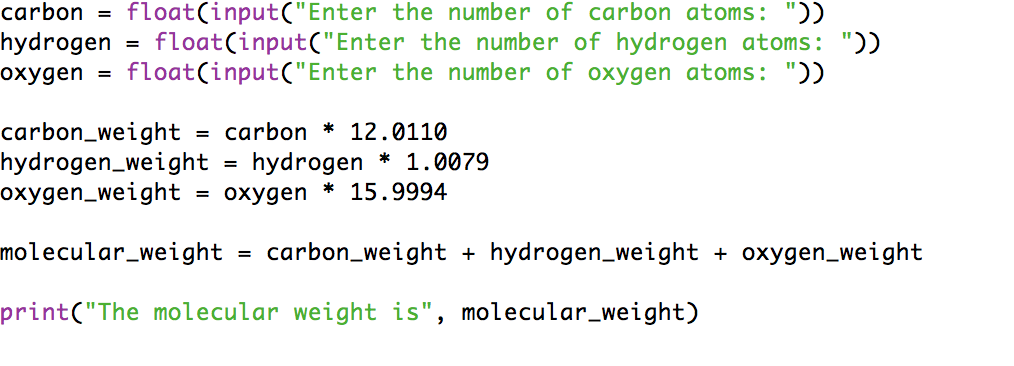
Previous program looked like this:
5.14 Enhanced molecular weight program (1) 6 points. Revise your program for the molecular weight of a chemical compound of hydrogen, carbon, and oxygen, so that it can take as user input a chemical formula of the form C2H50H for ethyl alcohol, COOHC6H40COCH3 or C9H804 for aspirin, CH3CH2CH2CH2CH2CH2CH2CH3 or C8H18 for octane, and so forth. You should write an algorithm to analyse an input string of this form, using string indexing as in section 3.1 to look at individual characters in the string. You should accumulate the total counts of hydrogen, carbon, and oxygen in the compound, and then use the formula in your previous section 2.11 assignment to compute the molecular weight. l suggest you use list for these three counts, and increment the appropriate count as you process the input string. Be careful to deal with the case when there is no number following the letter, which means the implied number is just one, and the case, as for octane above, when the number after a letter has more than one digit. The program should keep outputting the prompt Enter a chemical formula, or just the enter key to quit: for the chemical formula of a new compound, until the user presses only the enter key without a chemical formula, in which case the program should terminate. The molecular weights should be printed with exactly four digits after the decimal point (since the atomic weights from section 2.11 have four decimal places) Below is my output for the four chemical formulas above. The user input will not appear in the output used by the zyBooks comparison tests when grading. There are also some hidden test cases Enter a chemical formula, or just the enter key to quit: C2H5OH The molecular weight is 46.0688 Enter a chemical formula, or just the enter key to quit: COOHC6H4OCOCH3 The molecular weight is 180.1598 Enter a chemical formula, or just the enter key to quit: C9H804 The molecular weight is 180.1598 Enter a chemical formula, or just the enter key to quit: C8H18 The molecular weight is 114.2302 Enter a chemical formula, or enter key to quit: (2) 3 points. Modify your program so that if the user enters an invalid formula, for example, one that contains other characters besides digits and C, H, and O, or begins with a digit, the program will print the line "Badly formed molecular formula, try again. and then the input prompt again. So a possible session might look like Enter a chemical formula, or just the enter key to quit: 14H Badly formed molecular formula; try again. Enter a chemical formula, or just the enter key to quit: h14 Badly formed molecular Tormulai try again Enter a chemical formula, or just the enter key to quit: H14 The molecular weight is 14.1106 Enter a chemical formula, or the enter key to quit








