Answered step by step
Verified Expert Solution
Question
1 Approved Answer
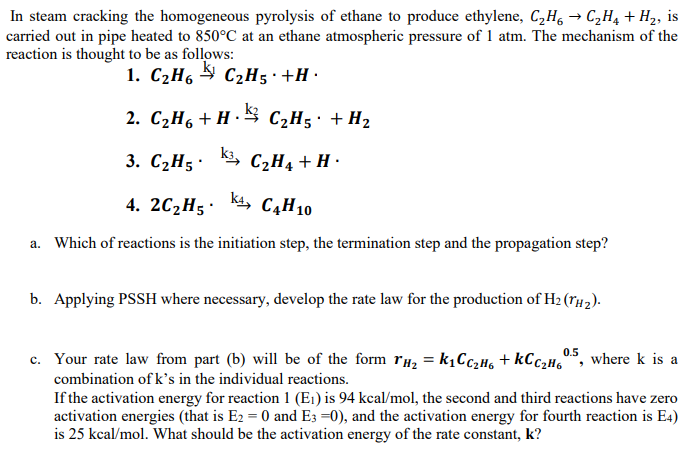
In steam cracking the homogeneous pyrolysis of ethane to produce ethylene, C 2 H 6 C 2 H 4 + H 2 , is carried
In steam cracking the homogeneous pyrolysis of ethane to produce ethylene, is
carried out in pipe heated to at an ethane atmospheric pressure of atm. The mechanism of the
reaction is thought to be as follows:
a Which of reactions is the initiation step, the termination step and the propagation step?
b Applying PSSH where necessary, develop the rate law for the production of
c Your rate law from part b will be of the form where is a
combination of s in the individual reactions.
If the activation energy for reaction is kca the second and third reactions have zero
activation energies that is and and the activation energy for fourth reaction is
is kca What should be the activation energy of the rate constant,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


