Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In the animation, you can see that the electrons occupy different orbitals according to the energy level of each orbital. A single box represents
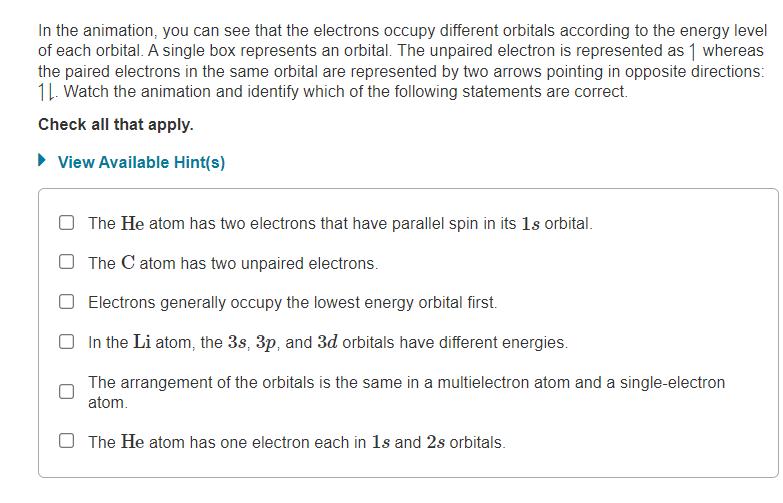
In the animation, you can see that the electrons occupy different orbitals according to the energy level of each orbital. A single box represents an orbital. The unpaired electron is represented as 1 whereas the paired electrons in the same orbital are represented by two arrows pointing in opposite directions: 1. Watch the animation and identify which of the following statements are correct. Check all that apply. View Available Hint(s) The He atom has two electrons that have parallel spin in its 1s orbital. The C atom has two unpaired electrons. Electrons generally occupy the lowest energy orbital first. In the Li atom, the 3s, 3p, and 3d orbitals have different energies. The arrangement of the orbitals is the same in a multielectron atom and a single-electron atom. The He atom has one electron each in 1s and 2s orbitals.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


