Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In the combustion of Hydrogen, the following reactions involving radicals are fast in both the forward and backward directions K H+O OH+O KD k2f
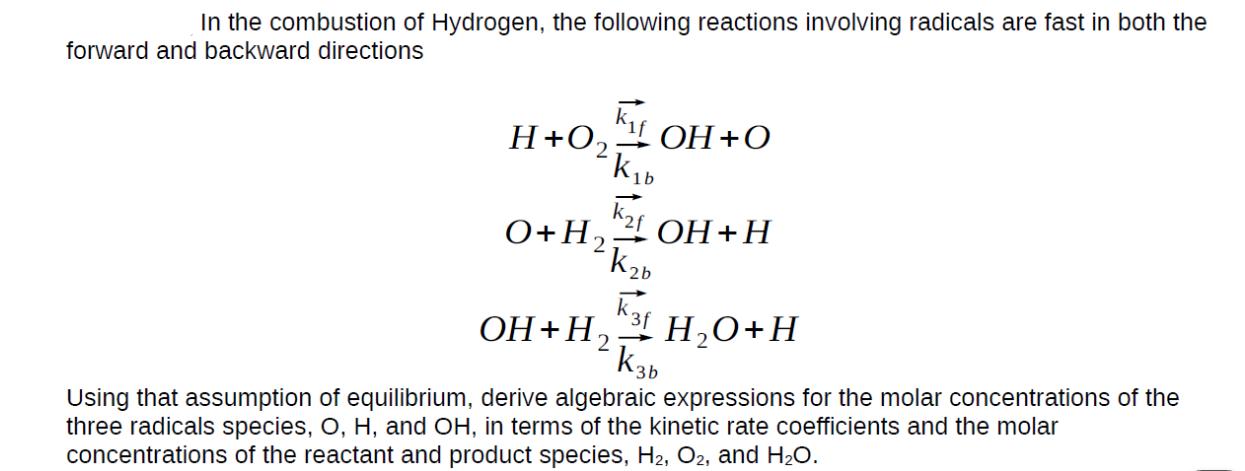
In the combustion of Hydrogen, the following reactions involving radicals are fast in both the forward and backward directions K H+O OH+O KD k2f O + H2 OH+H Kb HO+H OH+H K3b Using that assumption of equilibrium, derive algebraic expressions for the molar concentrations of the three radicals species, O, H, and OH, in terms of the kinetic rate coefficients and the molar concentrations of the reactant and product species, H2, O2, and HO.
Step by Step Solution
★★★★★
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
To derive the algebraic expressions for the molar concentrations of the radical species O H and OH in terms of the kinetic rate coefficients and the molar concentrations of the reactant and product sp...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


