Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In the Deacon process for the manufacture of C12, a dry mixture of HCl and air is passed over a heated catalyst that promotes
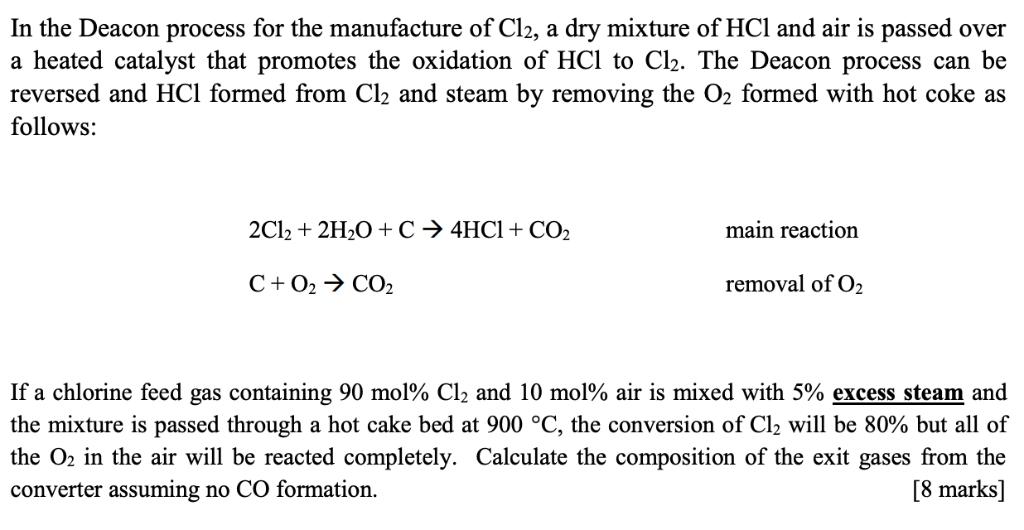
In the Deacon process for the manufacture of C12, a dry mixture of HCl and air is passed over a heated catalyst that promotes the oxidation of HCl to Cl2. The Deacon process can be reversed and HCl formed from Cl2 and steam by removing the O2 formed with hot coke as follows: 2Cl2 + 2H2O + C 4HC1+ CO2 main reaction C+ O2 CO2 removal of O2 If a chlorine feed gas containing 90 mol% Cl and 10 mol% air is mixed with 5% excess steam and the mixture is passed through a hot cake bed at 900 C, the conversion of Cl will be 80% but all of the O2 in the air will be reacted completely. Calculate the composition of the exit gases from the converter assuming no CO formation. [8 marks]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


