Answered step by step
Verified Expert Solution
Question
1 Approved Answer
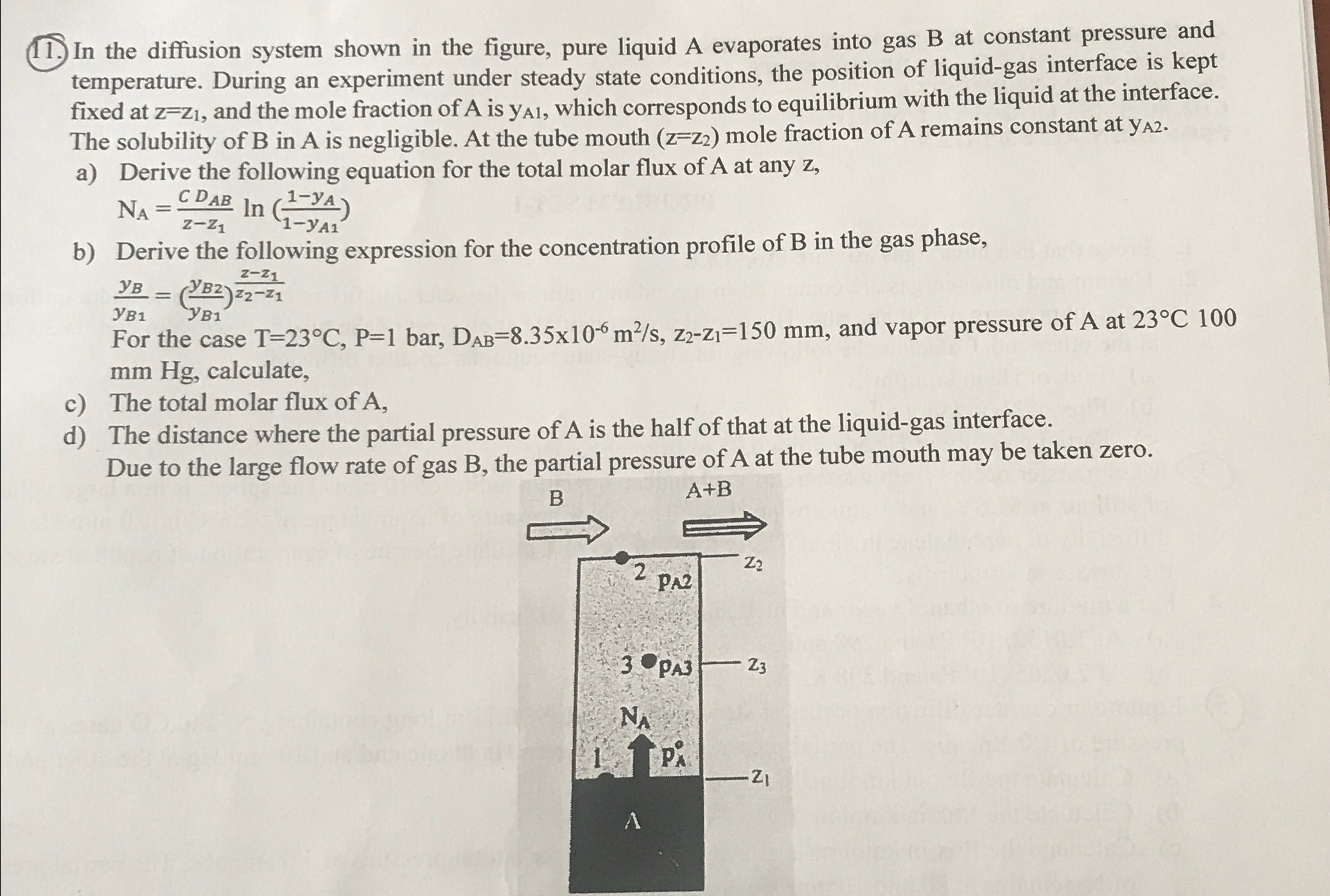
In the diffusion system shown in the figure, pure liquid A evaporates into gas B at constant pressure and temperature. During an experiment under steady
In the diffusion system shown in the figure, pure liquid A evaporates into gas B at constant pressure and temperature. During an experiment under steady state conditions, the position of liquidgas interface is kept fixed at and the mole fraction of is which corresponds to equilibrium with the liquid at the interface. The solubility of in is negligible. At the tube mouth mole fraction of A remains constant at
a Derive the following equation for the total molar flux of at any
b Derive the following expression for the concentration profile of in the gas phase,
For the case and vapor pressure of at calculate,
c The total molar flux of
d The distance where the partial pressure of is the half of that at the liquidgas interface.
Due to the large flow rate of gas the partial pressure of at the tube mouth may be taken zero.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


