Question
. In the fabrication of semiconductors, one step is the formation of additional gallium arsenide (GaAs) on the surface of a GaAs wafer. To accomplish
. In the fabrication of semiconductors, one step is the formation of additional gallium arsenide (GaAs) on the surface of a GaAs wafer. To accomplish this, gaseous tri- methyl gallium (TMG) and arsine flow in a carrier gas (usually hydrogen) over the solid and react on the solid surface at a temperature of 900K to produce the solid GaAs and gaseous methane. The unreacted TMG and arsine and the newly produced methane flow out in the outlet gas.
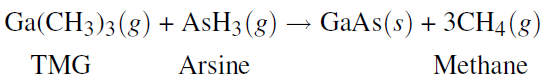
This process can be treated as a steady-state process wherein the deposition of the solid is treated as an outlet stream of pure solid GaAs. For such a process, the following are given:
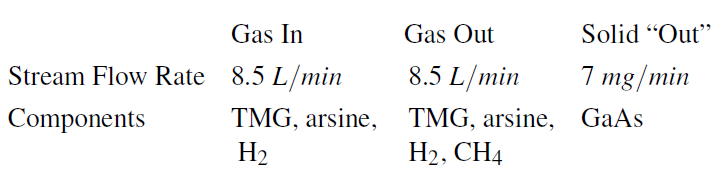
where the quantity in the solid out indicates that we wish to deposit 7 mg/min of GaAs. Also, we have assumed that the flow rate of the gas stream doesnt change significantly.
What is the concentration of methane in the outlet gas?
Ga(CH3)3(g)+AsH3(g)GaAs(s)+3CH4(g) TMG Arsine Methane Gas In Gas Out Solid "Out" Stream Flow Rate 8.5L/min8.5L/min7mg/min Components TMG, arsine, TMG, arsine, GaAs H2H2,CH4Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


