Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In the following reaction: KMNO4 + NazCz04 + H2SO4 + CO2 + MnSO4 + NazSO4 + K2SO4 + H2O What is the mean oxidation
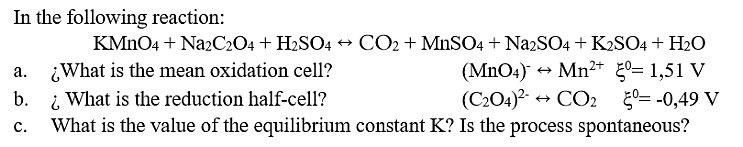
In the following reaction: KMNO4 + NazCz04 + H2SO4 + CO2 + MnSO4 + NazSO4 + K2SO4 + H2O What is the mean oxidation cell? i What is the reduction half-cell? What is the value of the equilibrium constant K? Is the process spontaneous? (MnO4) Mn+ 0= 1,51 V (C204)? + . b. + CO2 50= -0,49 V .
Step by Step Solution
★★★★★
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
clASSMate Solution Date Page The Reaction has two mode ones is oxidation and another reduction both ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


