Answered step by step
Verified Expert Solution
Question
1 Approved Answer
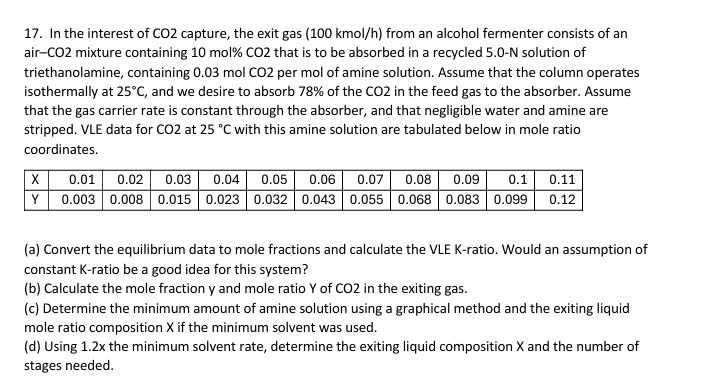
In the interest of C O 2 capture, the exit gas ( 1 0 0 kmo l h ) from an alcohol fermenter consists of
In the interest of capture, the exit gas kmo from an alcohol fermenter consists of an
airCO mixture containing mol that is to be absorbed in a recycled N solution of
triethanolamine, containing molCO per mol of amine solution. Assume that the column operates
isothermally at and we desire to absorb of the in the feed gas to the absorber Assume
that the gas carrier rate is constant through the absorber and that negligible water and amine are
stripped. VLE data for at with this amine solution are tabulated below in mole ratio
coordinates.
a Convert the equilibrium data to mole fractions and calculate the VLE Kratio. Would an assumption of
constant Kratio be a good idea for this system?
b Calculate the mole fraction and mole ratio of in the exiting gas.
c Determine the minimum amount of amine solution using a graphical method and the exiting liquid
mole ratio composition if the minimum solvent was used.
d Using the minimum solvent rate, determine the exiting liquid composition and the number of
stages needed.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


