Answered step by step
Verified Expert Solution
Question
1 Approved Answer
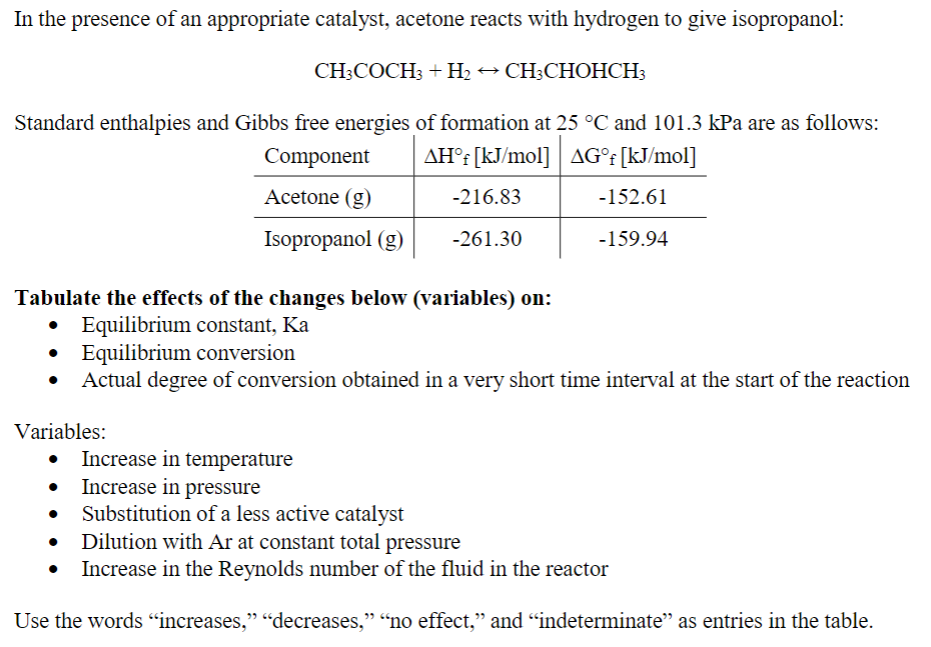
In the presence of an appropriate catalyst, acetone reacts with hydrogen to give isopropanol: C H 3 C O C H 3 + H 2
In the presence of an appropriate catalyst, acetone reacts with hydrogen to give isopropanol:
Standard enthalpies and Gibbs free energies of formation at and kPa are as follows:
Tabulate the effects of the changes below variables on:
Equilibrium constant,
Equilibrium conversion
Actual degree of conversion obtained in a very short time interval at the start of the reaction
Variables:
Increase in temperature
Increase in pressure
Substitution of a less active catalyst
Dilution with Ar at constant total pressure
Increase in the Reynolds number of the fluid in the reactor
Use the words "increases," "decreases," no effect," and "indeterminate" as entries in the table.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


