Question
In the PV diagram below showing an isobaric expansion (constant pressure) and an isochoric (constant volume) process on an ideal monatomic gas, P=10x105Pa and
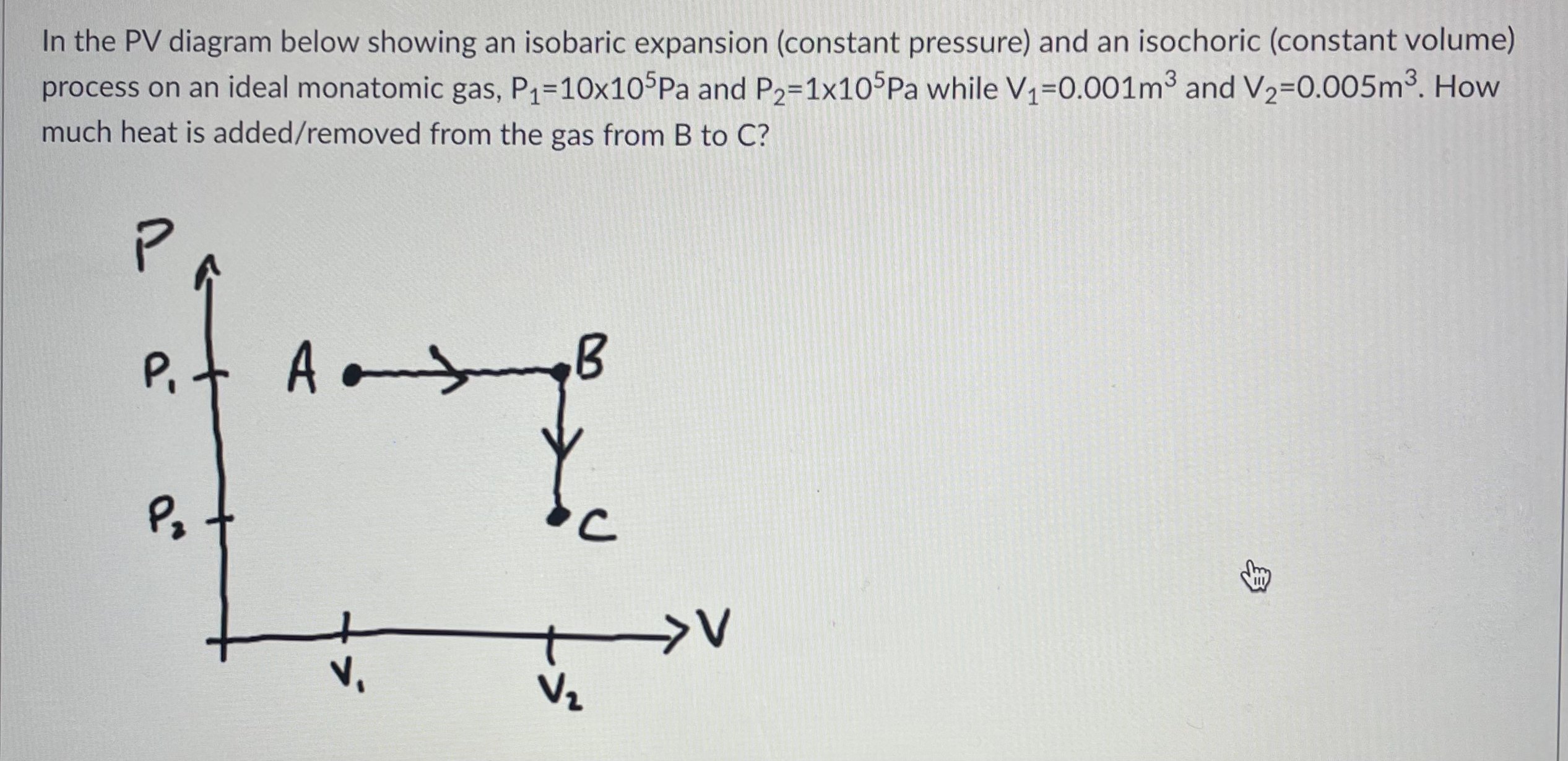
In the PV diagram below showing an isobaric expansion (constant pressure) and an isochoric (constant volume) process on an ideal monatomic gas, P=10x105Pa and P2=1x105Pa while V1=0.001m and V2=0.005m. How much heat is added/removed from the gas from B to C? P P Am P V C >V
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Biochemical And Engineering Thermodynamics
Authors: Stanley I. Sandler
5th Edition
047050479X, 978-0470504796
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



