Question
The burette is prepared with the standardized 1M HCI solution. A 25 ml aliquot of the Unknown is taken, transferred to an Erlenmeyer flask
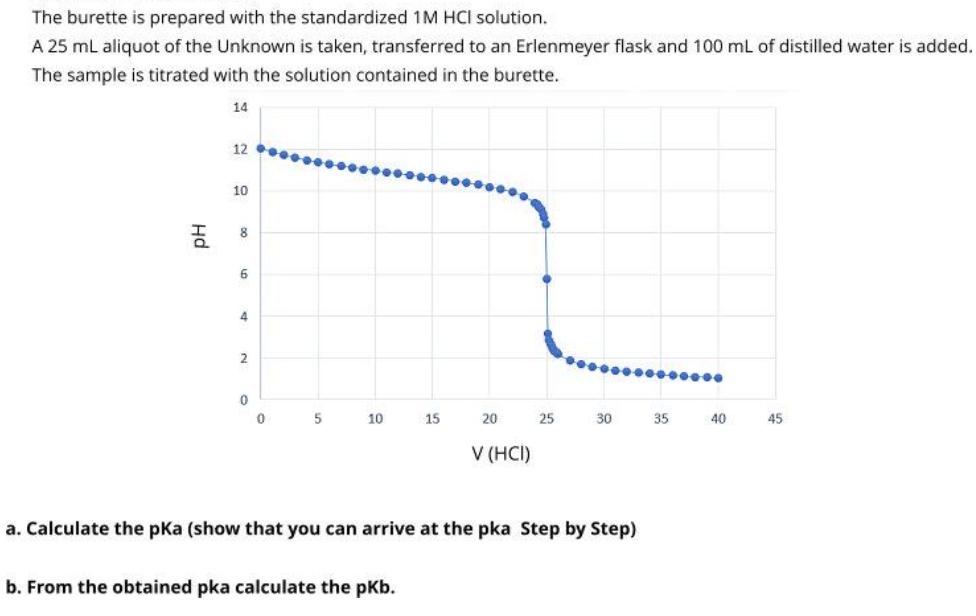
The burette is prepared with the standardized 1M HCI solution. A 25 ml aliquot of the Unknown is taken, transferred to an Erlenmeyer flask and 100 mL of distilled water is added. The sample is titrated with the solution contained in the burette. 14 12 10 6 4 2 10 15 20 25 30 35 40 45 V (HCI) a. Calculate the pKa (show that you can arrive at the pka Step by Step) b. From the obtained pka calculate the pKb. Hd
Step by Step Solution
3.34 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



