Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In this problem you will use the principle of time temperature superposition ( TTS ) to shift data ( G ' vs frequency ) for
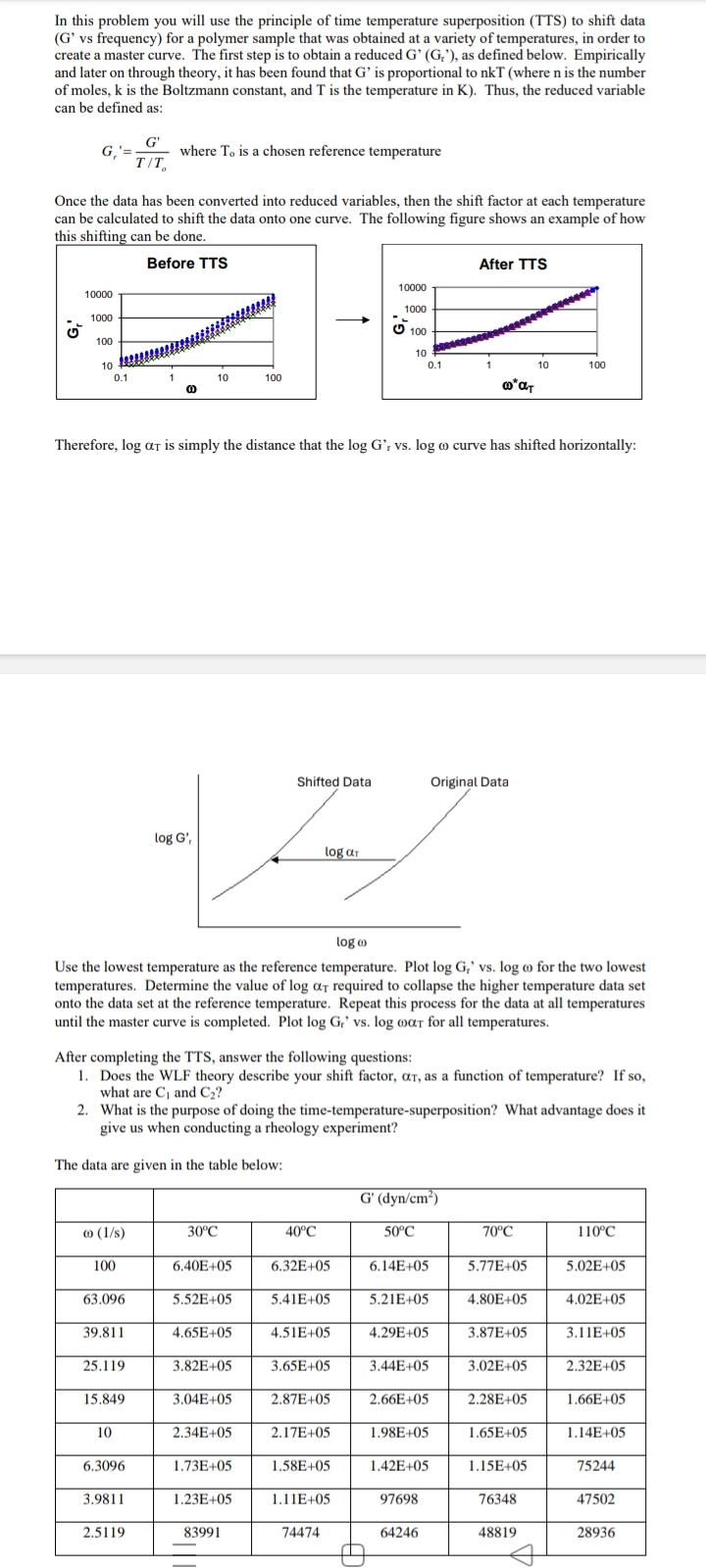
In this problem you will use the principle of time temperature superposition TTS to shift data G vs frequency for a polymer sample that was obtained at a variety of temperatures, in order to create a master curve. The first step is to obtain a reduced as defined below. Empirically and later on through theory, it has been found that is proportional to where is the number of moles, is the Boltzmann constant, and is the temperature in Thus, the reduced variable can be defined as:
where a chosen reference temperature
Once the data has been converted into reduced variables, then the shift factor at each temperature can be calculated to shift the data onto one curve. The following figure shows an example of how thie chiftine san he dono
Therefore, is simply the distance that the curve has shifted horizontally:
Use the lowest temperature as the reference temperature. Plot for the two lowest temperatures. Determine the value of required to collapse the higher temperature data set onto the data set at the reference temperature. Repeat this process for the data all temperatures until the master curve is completed. Plot for all temperatures.
After completing the TTS answer the following questions:
Does the WLF theory describe your shift factor, as a function of temperature? If so what are and
What is the purpose of doing the timetemperaturesuperposition? What advantage does it give us when conducting a rheology experiment?
The data are given in the table below:
table
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


