Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In this titration orange juice is used as the acid in place of HCI. That is because most fruit juices are acidic, with citric
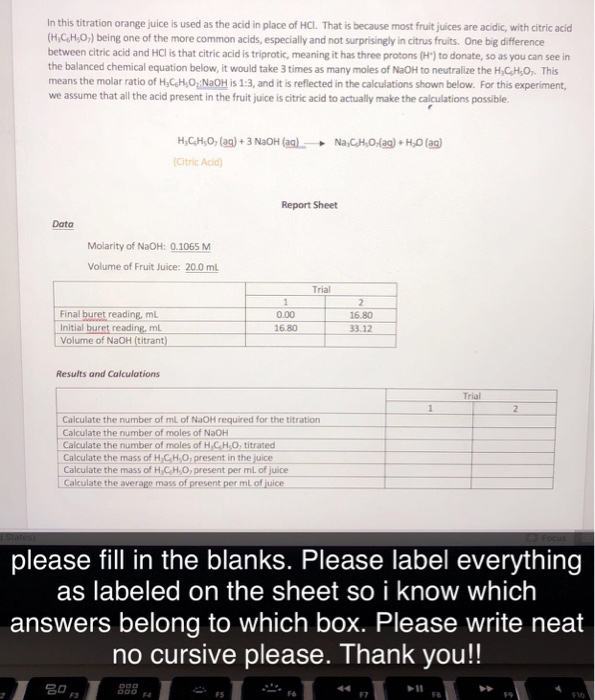
In this titration orange juice is used as the acid in place of HCI. That is because most fruit juices are acidic, with citric acid (H,C,H,O,) being one of the more common acids, especially and not surprisingly in citrus fruits. One big difference between citric acid and HCI is that citric acid is triprotic, meaning it has three protons (H) to donate, so as you can see in the balanced chemical equation below, it would take 3 times as many moles of NaOH to neutralize the H,C,H,O,. This means the molar ratio of HCHO NaOH is 1:3, and it is reflected in the calculations shown below. For this experiment, we assume that all the acid present in the fruit juice is citric acid to actually make the calculations possible. Data Final buret reading, ml Initial buret reading, ml Volume of NaOH (titrant) Molarity of NaOH: 0.1065 M Volume of Fruit Juice: 20.0 mL Results and Calculations HC,H,O, (ag) + 3 NaOH (ag) Na,C,H,O,(ag) + HO (ag) (Citric Acid) 80,5 Report Sheet 000 500 F4 1 0.00 16.80 Calculate the number of mL of NaOH required for the titration Calculate the number of moles of NaOH Calculate the number of moles of H,C,H,O, titrated Calculate the mass of H,C,H,O, present in the juice Calculate the mass of H,C,H,O, present per mL of juice Calculate the average mass of present per mL of juice Trial 2 16.80 33.12 1 Trial please fill in the blanks. Please label everything as labeled on the sheet so i know which answers belong to which box. Please write neat no cursive please. Thank you!! 2
Step by Step Solution
★★★★★
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Answer 7 Nant 01065M Juice 20me calculate the no of moles of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


