Answered step by step
Verified Expert Solution
Question
1 Approved Answer
in this whole question, the compound to be considered in all parts of the question is Ethanol 1. Write the balanced chemical equation for the
in this whole question, the compound to be considered in all parts of the question is Ethanol

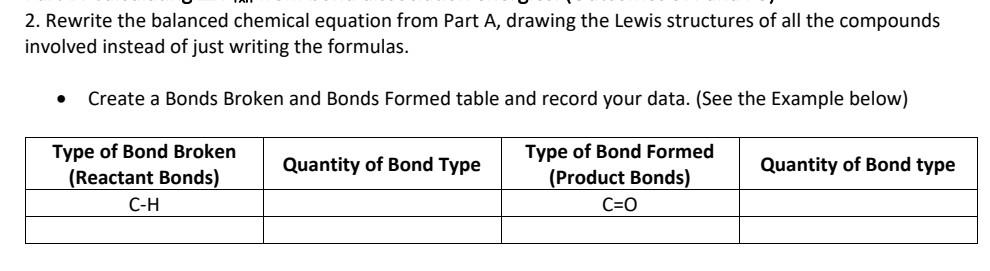

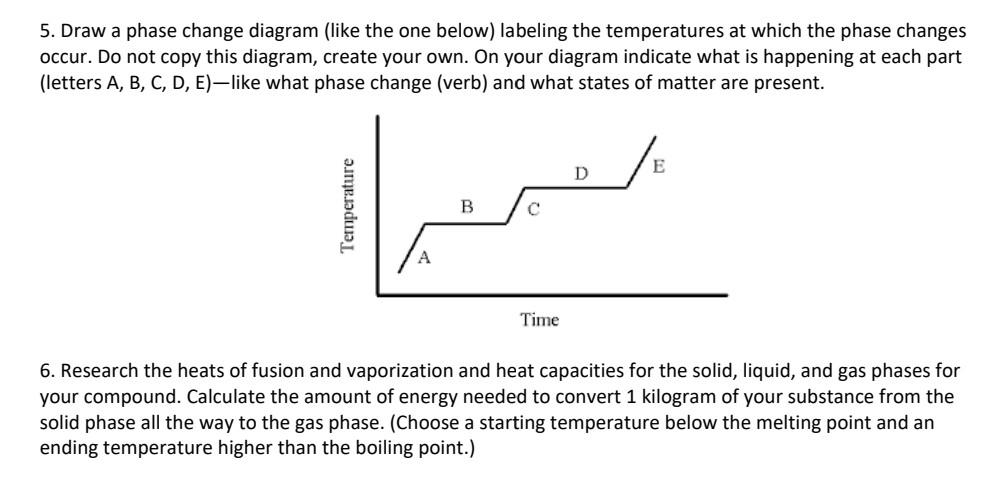

1. Write the balanced chemical equation for the combustion of your compound. From the equation and the values from Appendix G of your text, calculate the H for this reaction (assume standard conditions). - You will probably need to research the Hf for your particular compound. At the end of this document are some good sites with comprehensive thermochemical data. - If your compound has nitrogen in it, please add nitrogen gas (is it a HOFBrINCl?) to the products. If your compound has chlorine or fluorine in it, please add these elemental gases (are they HOFBrINCI?) to the products. 2. Rewrite the balanced chemical equation from Part A, drawing the Lewis structures of all the compounds involved instead of just writing the formulas. - Create a Bonds Broken and Bonds Formed table and record your data. (See the Example below) 3. Using the Bond Energies, calculate the Hrrn for the combustion reaction. You can find a chart of Bond Dissociation Energies in Section 7.5 (Tables 7.2 and 7.3) of your text. Section 7.5 also has some examples of this type of calculation. - Clearly show your work. It may be easier to show your work by modifying your chart in 2 (adding in the bond energy values and the total H from each compound) - Hrxn=H (bonds broken) H (bonds formed) 4. Compare the values from Parts A and B. Try to account for any differences. - If you have significant differences, which calculation do you think is more accurate? 5. Draw a phase change diagram (like the one below) labeling the temperatures at which the phase changes occur. Do not copy this diagram, create your own. On your diagram indicate what is happening at each part (letters A, B, C, D, E)-like what phase change (verb) and what states of matter are present. 6. Research the heats of fusion and vaporization and heat capacities for the solid, liquid, and gas phases for your compound. Calculate the amount of energy needed to convert 1 kilogram of your substance from the solid phase all the way to the gas phase. (Choose a starting temperature below the melting point and an ending temperature higher than the boiling point.) 7. Would your compound be a good choice to be used as a fuel? rocket fuel? airplane fuel? - Using your value from Part A-determine the energy density of your compound. (kJ/gram) - How does this energy density compare to other fuels? - Choose two commonly used fuels and compare. 8. Would your compound be a good choice to be used as a coolant? Why or why not? - Use your work from Part C to support your answer. - Compare your compound to two typical coolants. How do your compound's properties compare to common coolants? - What are qualities of "good" coolants? - In what engineering applications are coolants needed/used
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


