Question
Include graphs where necessary. Kindly solve both the subparts of the problem. I will give like after getting my requirements. thank you. Do not copy-paste
Include graphs where necessary.
Kindly solve both the subparts of the problem. I will give like after getting my requirements. thank you.
Do not copy-paste anything from google or any previous solves. kindly write in your own language.
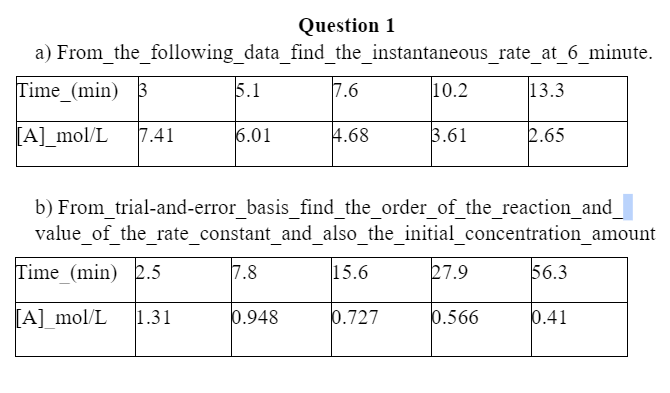
For question-(b),
You have to show the graph for zero, first, second, and third-order reactions. The graph at which you get the straight line will indicate the order, then you can conclude the data are not for that particular order. Through this trial and error process, You have to select the order, and then you will do the rest of the math.
Question 1 a) From_the_following_data_find_the_instantaneous_rate_at_6_minute. Time (min) 5 5.1 7.6 10.2 13.3 [A]_mol/L 7.41 6.01 4.68 3.61 2.65 b) From_trial-and-error_basis_find_the_order_of_the_reaction and value_of_the_rate_constant_and_also_the_initial_concentration amount Time (min) 2.5 17.8 15.6 27.9 56.3 [A] mol/L 1.31 0.948 0.727 0.566 0.41Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


