Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Increasing Temperature (C) The designed submarine is intended to operate in the maximum depth of around 500m. According to the temperature-depth ocean water profile
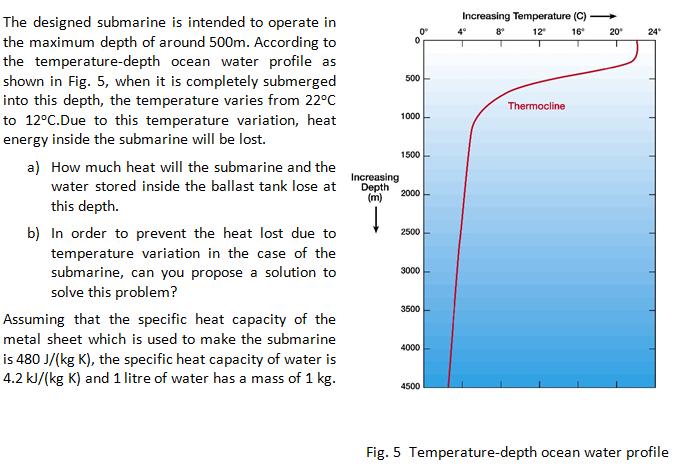
Increasing Temperature (C) The designed submarine is intended to operate in the maximum depth of around 500m. According to the temperature-depth ocean water profile as shown in Fig. 5, when it is completely submerged into this depth, the temperature varies from 22C to 12C.Due to this temperature variation, heat energy inside the submarine will be lost. 8 12 16 20 24 500 Thermocline 1000 1500 a) How much heat will the submarine and the Increasing Depth (m) water stored inside the ballast tank lose at 2000 this depth. b) In order to prevent the heat lost due to 2500 temperature variation in the case of the submarine, can you propose a solution to solve this problem? 3000 3500 Assuming that the specific heat capacity of the metal sheet which is used to make the submarine 4000 is 480 J/(kg K), the specific heat capacity of water is 4.2 kJ/(kg K) and 1 litre of water has a mass of 1 kg. 4500 Fig. 5 Temperature-depth ocean water profile
Step by Step Solution
★★★★★
3.39 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


