Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An FCC iron-carbon alloy has an initial uniform carbon concentration of 0.25 wt% C. The alloy is carburized at 950 C and in an
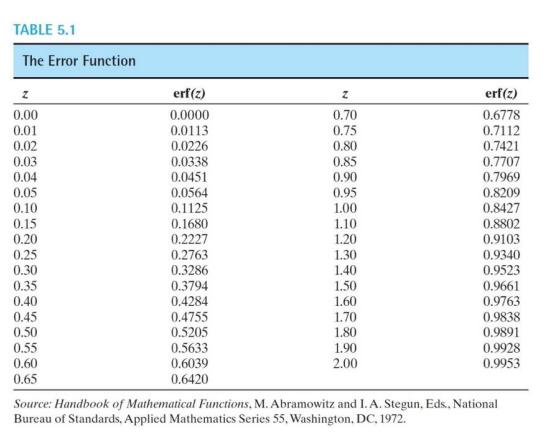
An FCC iron-carbon alloy has an initial uniform carbon concentration of 0.25 wt% C. The alloy is carburized at 950 C and in an atmosphere that gives a constant surface carbon concentration constant of 1.2 wt.% a) How much time is needed to get a carbon concentration of 0.8 wt% at a position 0.4 mm below the surface? (5 marks) b) How much time is needed to get the same carbon concentration if the carburization takes place at 750 C? How does it compare to the time needed at 950 C? (3 marks) Interpolate the error function to obtain your solution. Assume that the diffusivity (D) is a function of temperature and follows this relation D=Doe^-(QRT) where, D. is the standard diffusivity of 2.3 x 10^-5 m, R is the gas constant, T is the temperature, and Q is the activation energy of 148 kJ/mol. TABLE 5.1 The Error Function z 0.00 0.01 0.02 0.03 0.04 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45 0.50 0.55 0.60 0.65 erf(z) 0.0000 0.0113 0.0226 0.0338 0.0451 0.0564 0.1125 0.1680 0.2227 0.2763 0.3286 0.3794 0.4284 0.4755 0.5205 0.5633 0.6039 0.6420 z 0.70 0.75 0.80 0.85 0.90 0.95 1.00 1.10 1.20 1.30 1.40 1.50 1.60 1.70 1.80 1.90 2.00 erf(z) 0.6778 0.7112 0.7421 0.7707 0.7969 0.8209 0.8427 0.8802 0.9103 0.9340 0.9523 0.9661 0.9763 0.9838 0.9891 0.9928 0.9953 Source: Handbook of Mathematical Functions, M. Abramowitz and I. A. Stegun, Eds., National Bureau of Standards. Applied Mathematics Series 55, Washington, DC, 1972.
Step by Step Solution
★★★★★
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Answer The problem can be solved by using Ficks second law of diffusion which describes the movement of mass over time due to a concentration gradient ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


