Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Normal rain has a pH of 5.6 due to dissolved atmospheric carbon dioxide at a current level of 400 ppm. Various models predict that

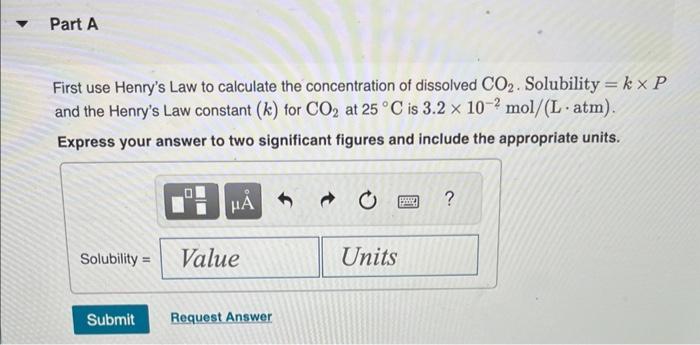
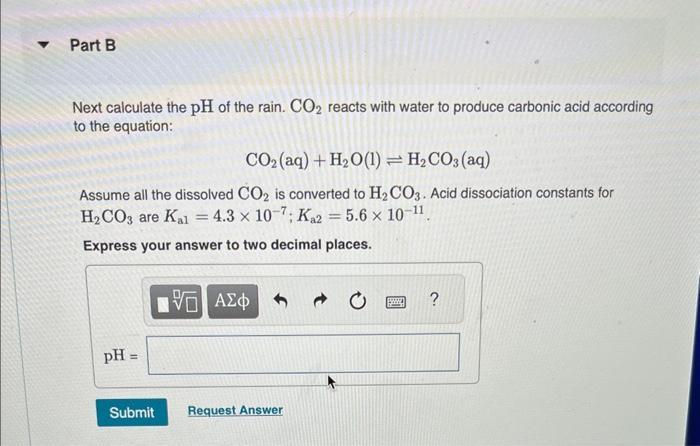
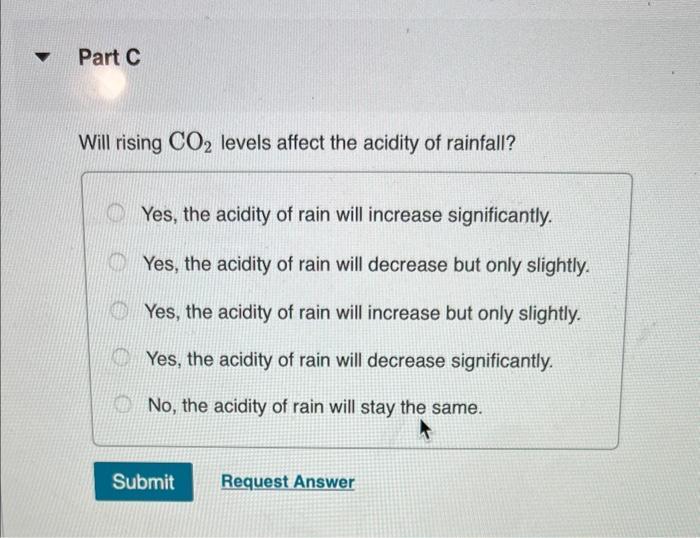
Normal rain has a pH of 5.6 due to dissolved atmospheric carbon dioxide at a current level of 400 ppm. Various models predict that burning fossil fuels will increase the atmospheric CO concentration to between 500 and 1000 ppm (by volume) by the year 2100. Calculate the pH of rain in a scenario where the CO concentration is 550 ppm. Part A First use Henry's Law to calculate the concentration of dissolved CO. Solubility = kx P and the Henry's Law constant (k) for CO2 at 25 C is 3.2 x 10-2 mol/(L atm). Express your answer to two significant figures and include the appropriate units. I Submit HA Solubility= Value Request Answer C Units P ? Part B Next calculate the pH of the rain. CO2 reacts with water to produce carbonic acid according to the equation: CO (aq) + HO(1) = HCO3(aq) Assume all the dissolved CO is converted to HCO3. Acid dissociation constants for HCO3 are Kal 4.3 x 10-7; K2 = 5.6 x 10-11. Express your answer to two decimal places. pH = Submit 15. Request Answer www Part C Will rising CO levels affect the acidity of rainfall? Yes, the acidity of rain will increase significantly. Yes, the acidity of rain will decrease but only slightly. Yes, the acidity of rain will increase but only slightly. Yes, the acidity of rain will decrease significantly. No, the acidity of rain will stay the same. Submit Request Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Part A Henrys Law allows us to figure out how much dissolved CO2 is in the rainfall Per Henrys Law solubility is equal to k P Where The concentration ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


