Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Introduction Consider the simple situation where we desire to cool a couple of six-packs of soda (or whatever your favorite beverage might be) by
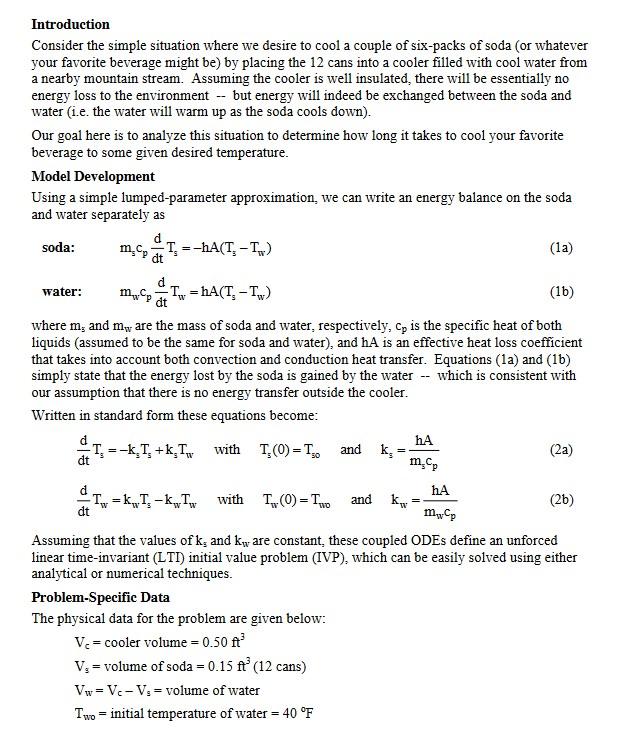


Introduction Consider the simple situation where we desire to cool a couple of six-packs of soda (or whatever your favorite beverage might be) by placing the 12 cans into a cooler filled with cool water from a nearby mountain stream. Assuming the cooler is well insulated, there will be essentially no energy loss to the environment -- but energy will indeed be exchanged between the soda and water (i.e. the water will warm up as the soda cools down). Our goal here is to analyze this situation to determine how long it takes to cool your favorite beverage to some given desired temperature. Model Development Using a simple lumped-parameter approximation, we can write an energy balance on the soda and water separately as soda: water: m.c, T=-hA(T-T) dt mcpTw=hA(T. - Tw) dt (1b) where m, and m, are the mass of soda and water, respectively, cp is the specific heat of both liquids (assumed to be the same for soda and water), and hA is an effective heat loss coefficient that takes into account both convection and conduction heat transfer. Equations (1a) and (1b) simply state that the energy lost by the soda is gained by the water -- which is consistent with our assumption that there is no energy transfer outside the cooler. Written in standard form these equations become: T(0) = 10 -T T-k, T+k,Twith = -T = KT-kwTw with dt = and Problem-Specific Data The physical data for the problem are given below: V = cooler volume = 0.50 ft V = volume of soda = 0.15 ft (12 cans) Vw = Vc - V = volume of water Two initial temperature of water = 40 F k, = hA m.cp hA mwCp Assuming that the values of k, and kw are constant, these coupled ODEs define an unforced linear time-invariant (LTI) initial value problem (IVP), which can be easily solved using either analytical or numerical techniques. (la) T. (0)=Two and kw = (2a) (26) Ts0 initial temperature of soda = 80 F = Ts5 = measured temperature of the soda after 5 minutes = 68 F Tsd = desired temperature of the soda 55 F p = density of both water and soda = 62.3 lbm/ft Cp = specific heat of both water and soda = 1.0 Btu/lbm-F Note that the measured soda temperature at t = 5 min will be used within an iterative simulation procedure to converge upon the numerical value of the hA product that gives the measured temperature value -- which then gives numerical values for the k, and kw constants. Thus, with the data given here, one should be able to determine the T.(t) and Tu(t) profiles and, in particular, determine how long it takes to cool the soda to the desired temperature of Tsd 55 F. Numerical Solution Solve this problem numerically using one of the built-in ODE solvers in Matlab (either the ode23 or ode45 routine, for example). For a pure numerical solution, you will have to solve for the needed ha value within an iterative procedure, where convergence is achieved when the guess for hA gives the measured value of T, at t = 5 minutes into the cooling process. You may do this by manually guessing hA within a looping structure or you can fully automate the iterative procedure -- your choice here. Once the iterative procedure is fully functional, plot the final converged Ts(t) and Tw(t) profiles as above and estimate the time it takes to cool the soda to the desired temperature of 55 F
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


