Answered step by step
Verified Expert Solution
Question
1 Approved Answer
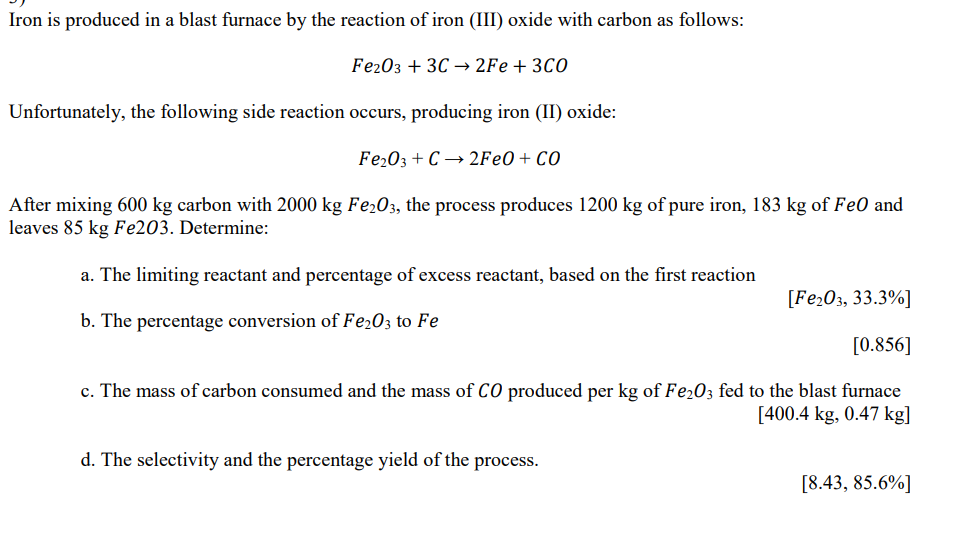
Iron is produced in a blast furnace by the reaction of iron ( III ) oxide with carbon as follows: F e 2 O 3
Iron is produced in a blast furnace by the reaction of iron III oxide with carbon as follows:
Unfortunately, the following side reaction occurs, producing iron II oxide:
FeO
After mixing carbon with the process produces of pure iron, of FeO and
leaves Fe Determine:
a The limiting reactant and percentage of excess reactant, based on the first reaction
b The percentage conversion of to
c The mass of carbon consumed and the mass of produced per of fed to the blast furnace
kg
d The selectivity and the percentage yield of the process.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


