Question
Write the net ionic equation for the equilibrium that is established when ammonium iodide is dissolved in water. (Use H3O+ instead of H+. It

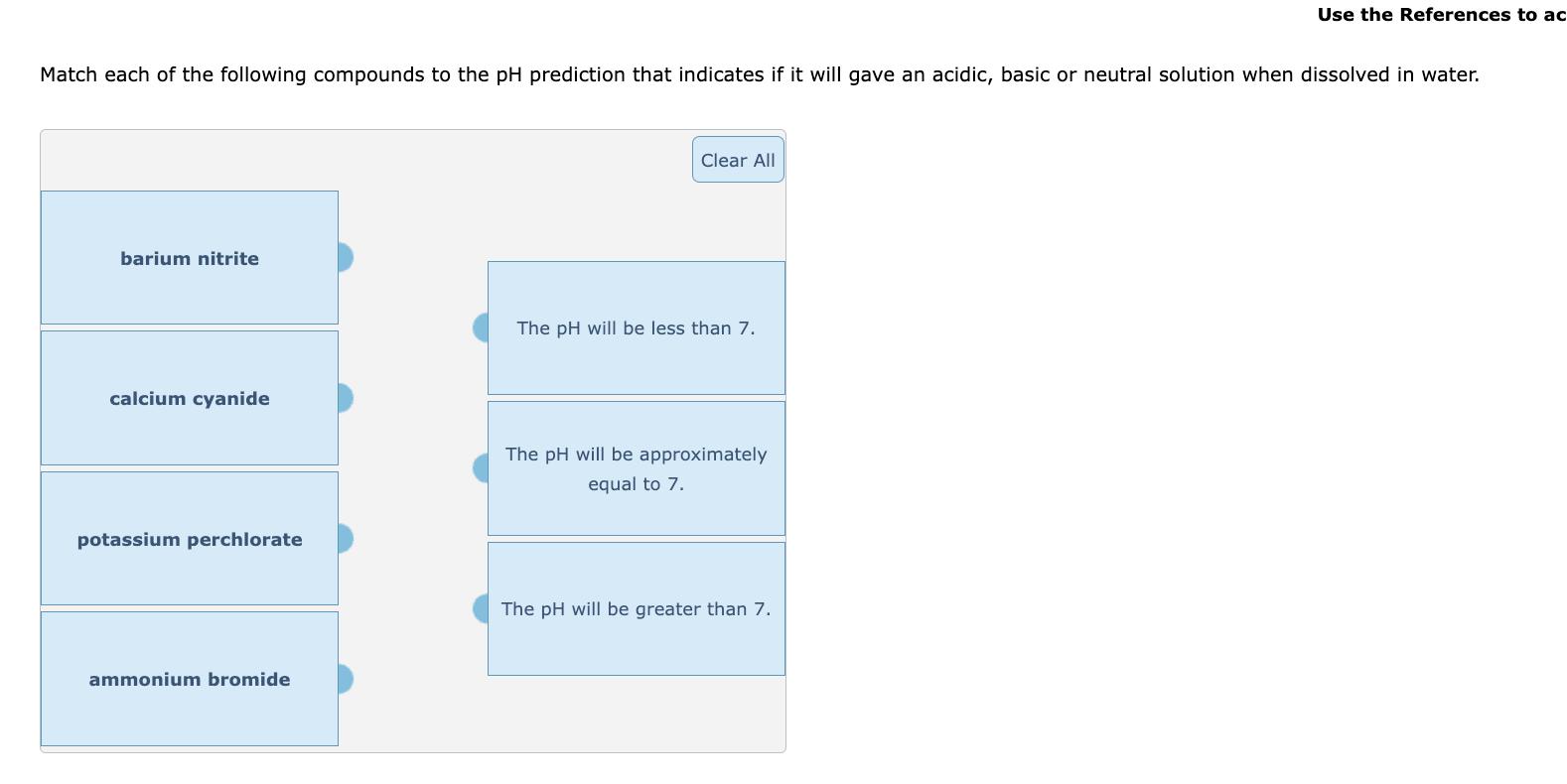
Write the net ionic equation for the equilibrium that is established when ammonium iodide is dissolved in water. (Use H3O+ instead of H+. It is not necessary to include states such as (aq) or (!).) + H2O This solution is + Use the References to ac Match each of the following compounds to the pH prediction that indicates if it will gave an acidic, basic or neutral solution when dissolved in water. barium nitrite Clear All The pH will be less than 7. calcium cyanide The pH will be approximately equal to 7. potassium perchlorate ammonium bromide The pH will be greater than 7.
Step by Step Solution
3.51 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 Given Acid base hydrolysis of NH4I in water Reaction NH4IaqH2OlNH4OHa...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



