Answered step by step
Verified Expert Solution
Question
1 Approved Answer
is this answer correct? The mass percent concentration of your solution expressed as %(m/m) is % (m/m) Data for Exp 14 Here is a copy
is this answer correct? 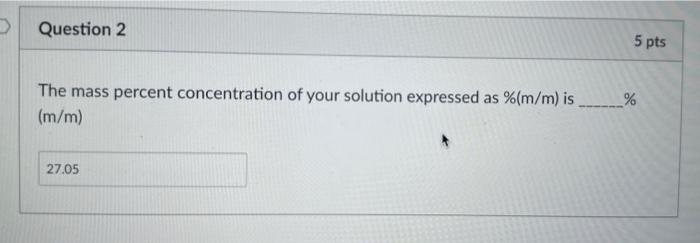
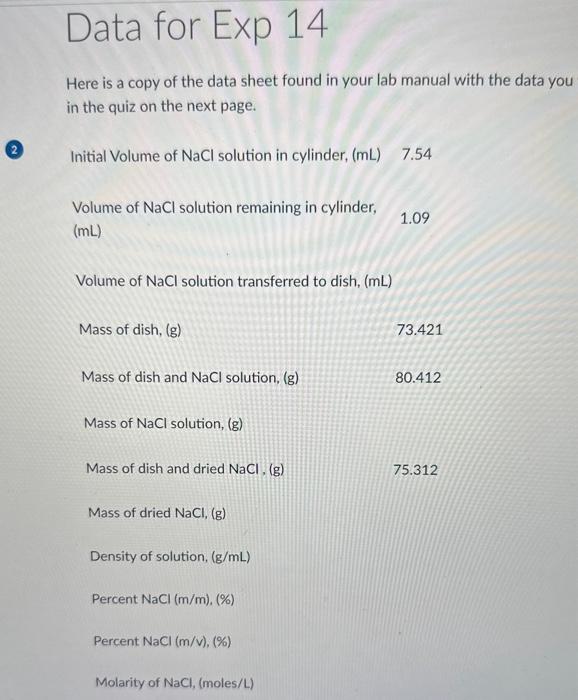
The mass percent concentration of your solution expressed as %(m/m) is % (m/m) Data for Exp 14 Here is a copy of the data sheet found in your lab manual with the data you in the quiz on the next page. (2) Initial Volume of NaCl solution in cylinder, (mL)7.54 Volume of NaCl solution remaining in cylinder, 1.09 (mL) Volume of NaCl solution transferred to dish, (mL) Mass of dish, (g) 73.421 Mass of dish and NaCl solution, ( g) 80.412 Mass of NaCl solution, (g) Mass of dish and dried NaCl. (g) 75.312 Mass of dried NaCl, (g) Density of solution, (g/mL) Percent NaCl(m/m), (\%) Percent NaCl(m/v),(%) Molarity of NaCl, (moles/L) 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


