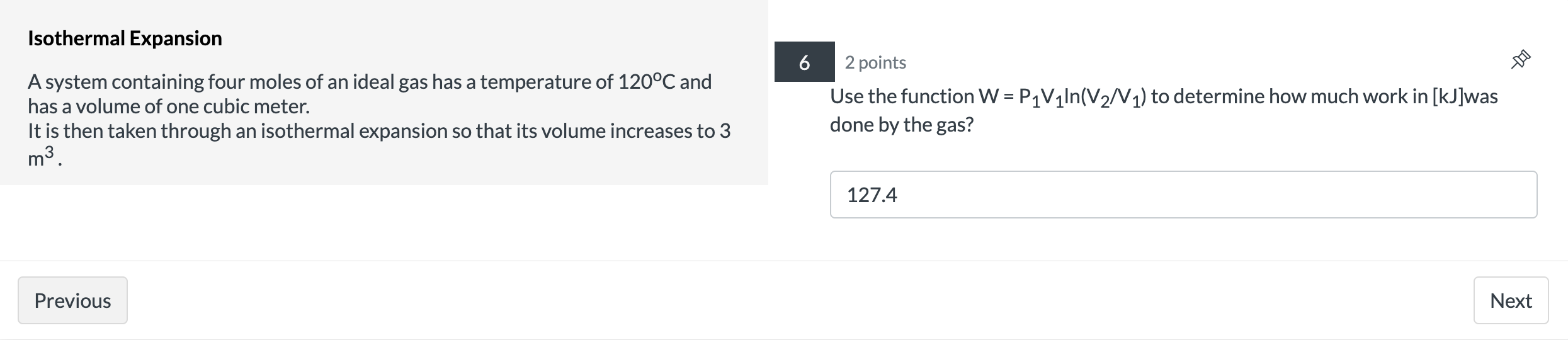
Question: Isothermal Expansion 2 points 9 A system containing four moles of an ideal gas has a temperature of 120C and has a volume of one
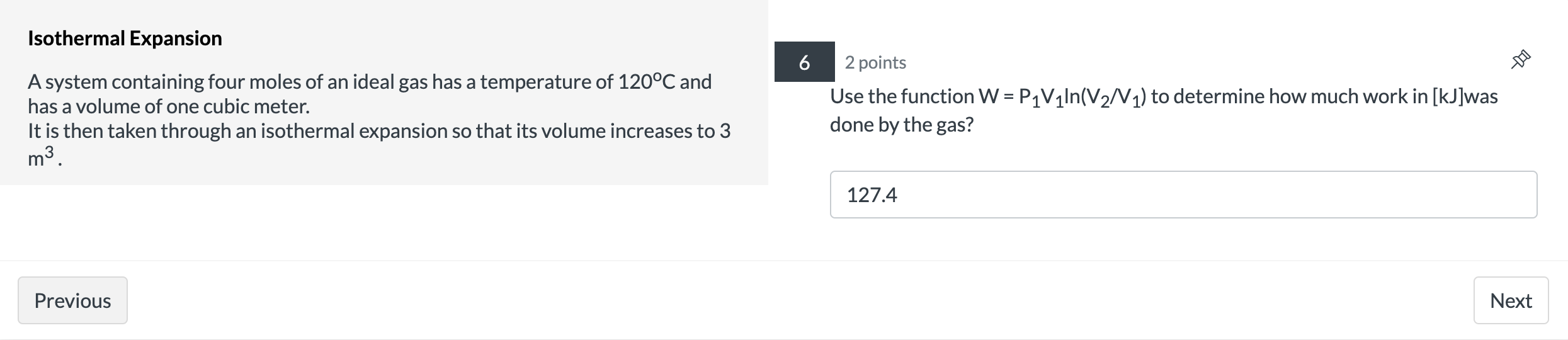
![[kJ]was 127.4 Previous Next Carnot Engine 7 1 point A Carnot engine](https://s3.amazonaws.com/si.experts.images/answers/2024/06/6676cdd631917_9746676cdd60d6ab.jpg)
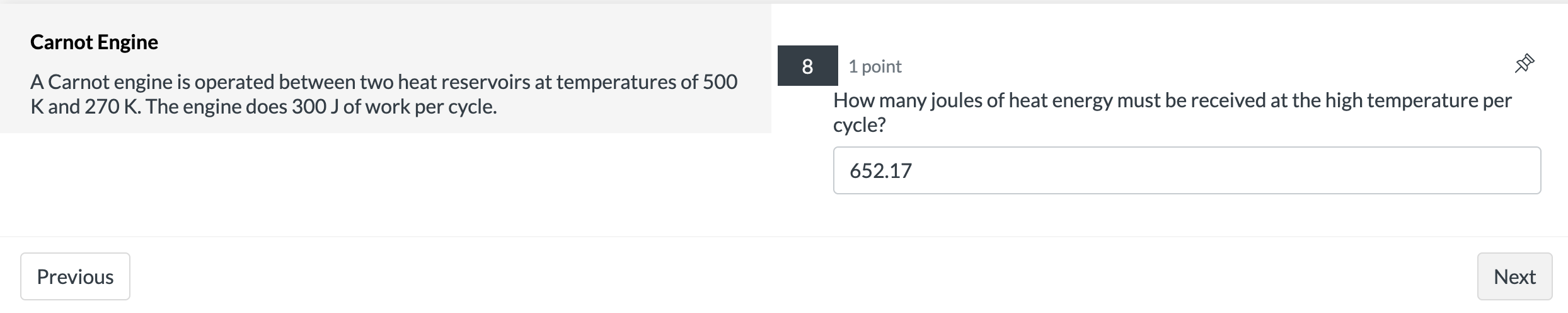
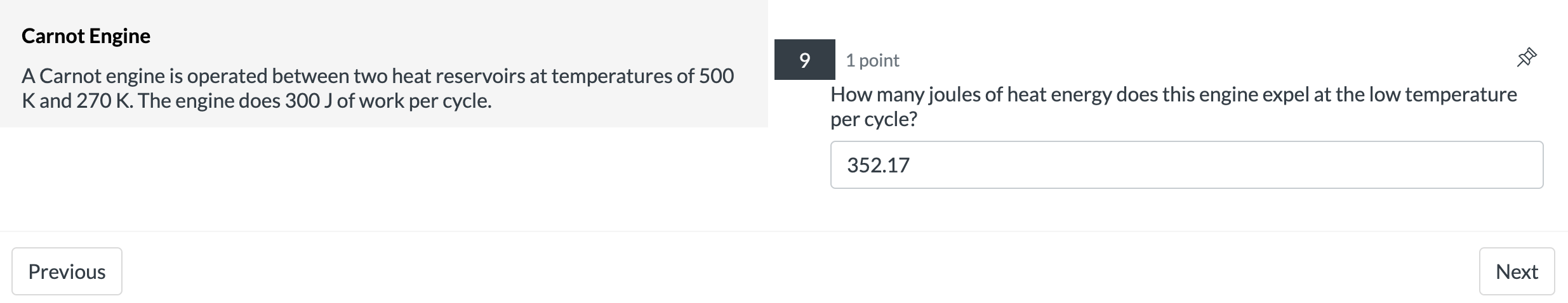
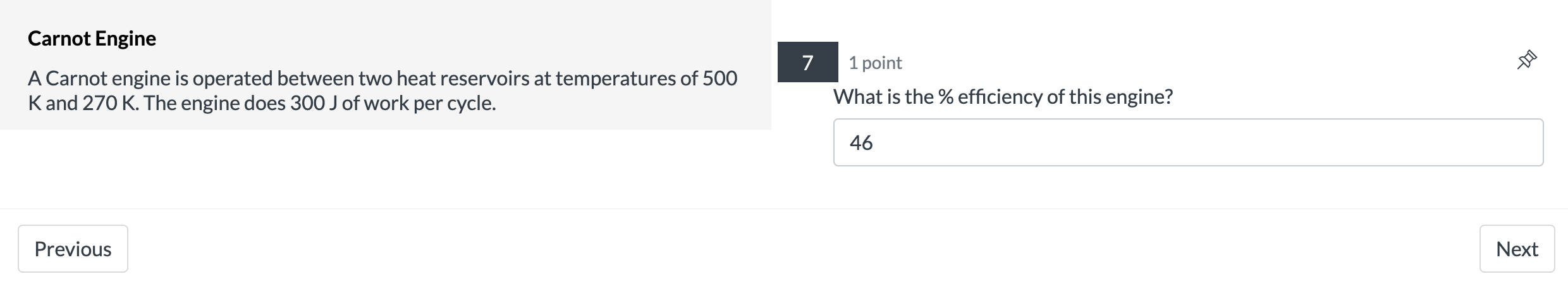
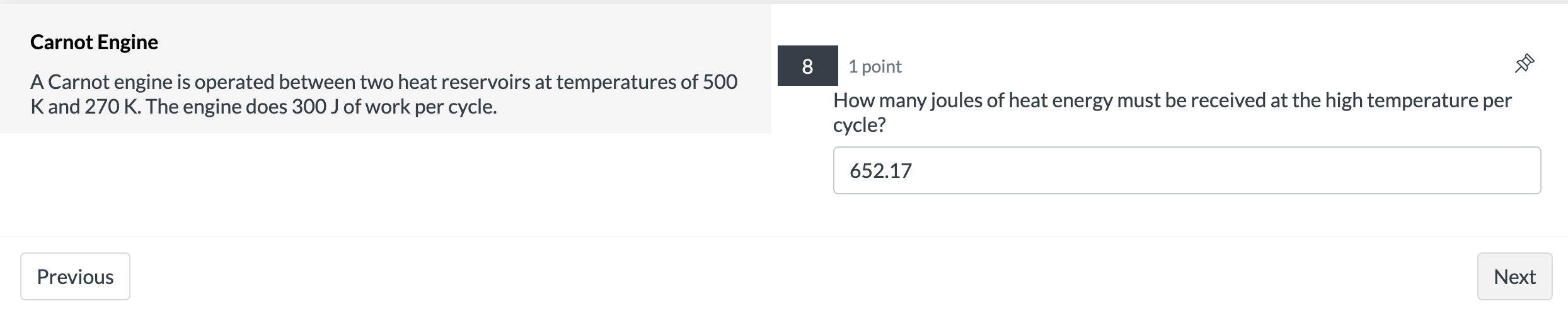
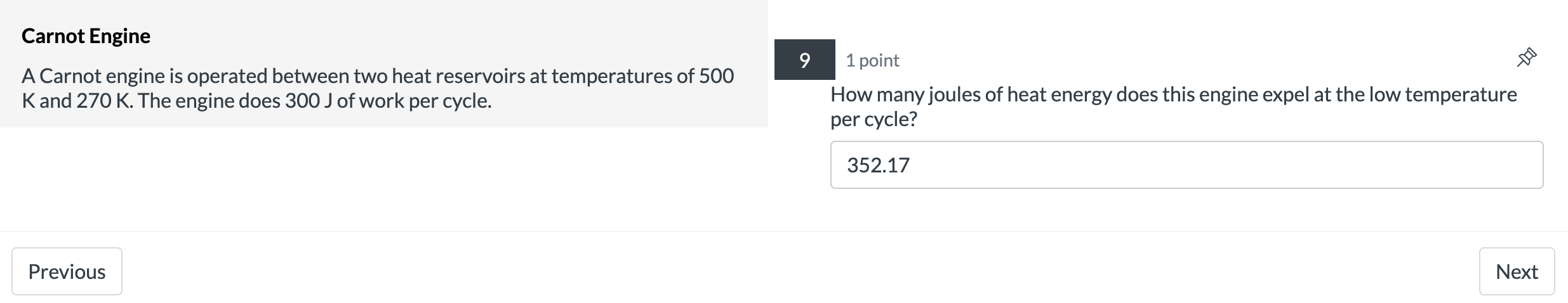
Isothermal Expansion 2 points 9 A system containing four moles of an ideal gas has a temperature of 120C and has a volume of one cubic meter. It is then taken through an isothermal expansion so that its volume increases to 3 done by the 335? m3. Use the function W = P1V1|n(V2N1) to determine how much work in [kJ]was 127.4 Previous Next Carnot Engine 7 1 point A Carnot engine is operated between two heat reservoirs at temperatures of 500 K and 270 K. The engine does 300 J of work per cycle. What is the % efficiency of this engine? 46 Previous NextCarnot Engine 8 1 point A Carnot engine is operated between two heat reservoirs at temperatures of 500 K and 270 K. The engine does 300 J of work per cycle. How many joules of heat energy must be received at the high temperature per cycle? 652.17 Previous NextCarnot Engine 9 1 point A Carnot engine is operated between two heat reservoirs at temperatures of 500 K and 270 K. The engine does 300 J of work per cycle. How many joules of heat energy does this engine expel at the low temperature per cycle? 352.17 Previous Next
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


