Question
It is commonly assumed that Z at standard conditions (psc = 14.7 psi and T=60F) is equal to 1. Show whether this is a
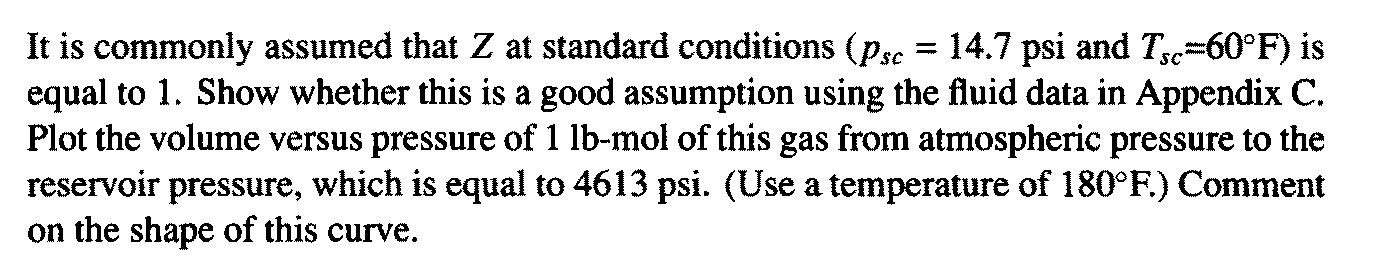
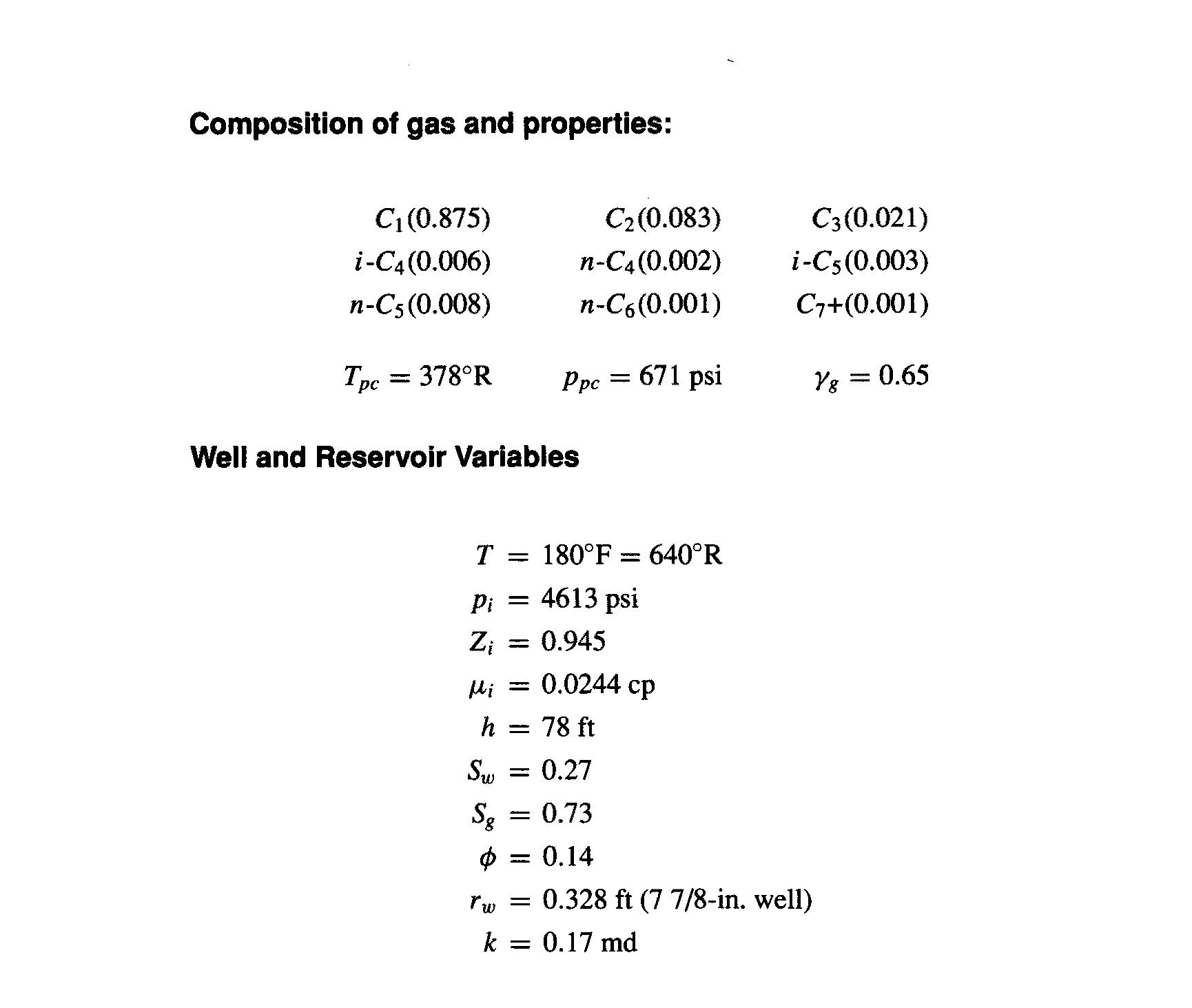
It is commonly assumed that Z at standard conditions (psc = 14.7 psi and T=60F) is equal to 1. Show whether this is a good assumption using the fluid data in Appendix C. Plot the volume versus pressure of 1 lb-mol of this gas from atmospheric pressure to the reservoir pressure, which is equal to 4613 psi. (Use a temperature of 180F.) Comment on the shape of this curve. Composition of gas and properties: C (0.875) C2 (0.083) C3(0.021) i-C4(0.006) -(0.002) i-C5(0.003) n-C5 (0.008) n-C6(0.001) C7+(0.001) Tpc = 378R Ppc = 671 psi Yg = 0.65 Well and Reservoir Variables T = 180F 640R Pi = 4613 psi = 0.945 Hi = 0.0244 cp h = 78 ft = 0.27 Sw S3 0.73 0.14 rw = 0.328 ft (7 7/8-in. well) k = 0.17 md
Step by Step Solution
3.38 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Formation volume factor is defined as the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Physics
Authors: Jearl Walker, Halliday Resnick
8th Extended edition
471758019, 978-0471758013
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



