Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It is desired to convert reactant A into product P. A laboratory reaction rate study was performed by carrying out the reaction and measuring the
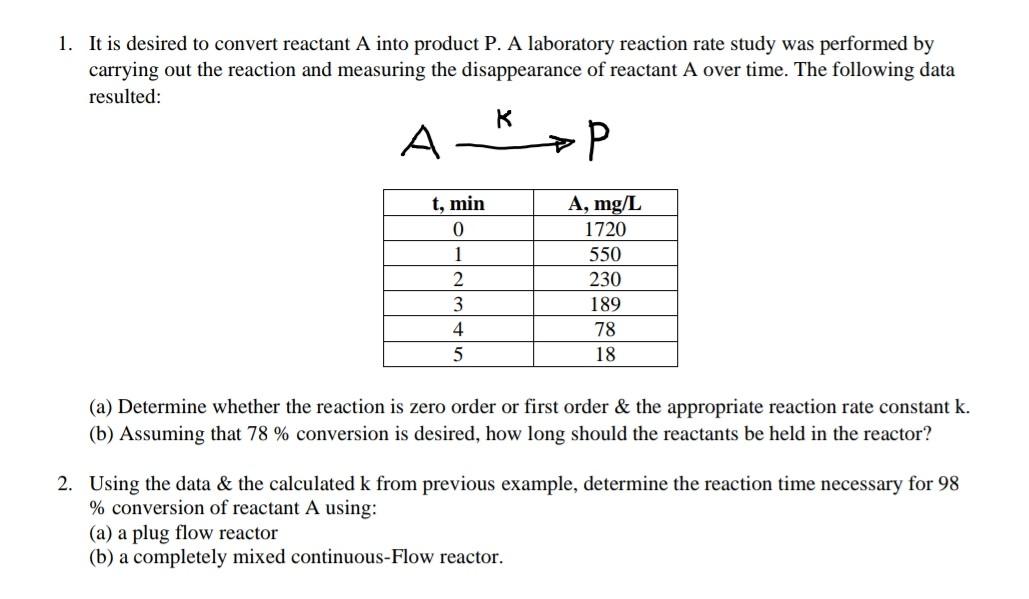
It is desired to convert reactant A into product P. A laboratory reaction rate study was performed by carrying out the reaction and measuring the disappearance of reactant A over time. The following data resulted: AkP (a) Determine whether the reaction is zero order or first order \& the appropriate reaction rate constant k. (b) Assuming that 78% conversion is desired, how long should the reactants be held in the reactor? Using the data \& the calculated k from previous example, determine the reaction time necessary for 98 % conversion of reactant A using: (a) a plug flow reactor (b) a completely mixed continuous-Flow reactor
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


