Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It is desired to design a reactor for the catalytic oxidation of formaldehyde (CH2O). The chemical reactions are: and The reaction must be carried out
It is desired to design a reactor for the catalytic oxidation of formaldehyde (CH2O).
The chemical reactions are:
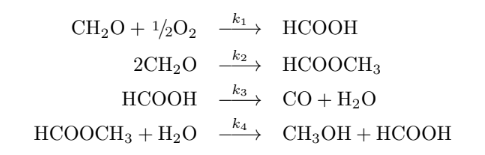
and
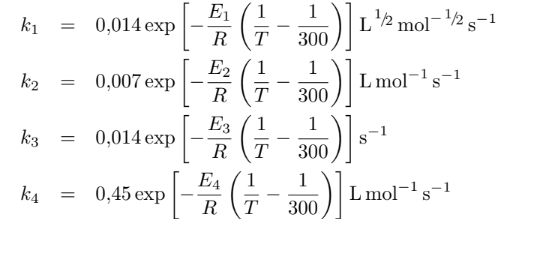
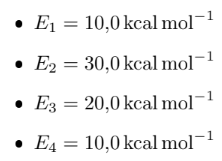
The reaction must be carried out in a tubular reactor. [1] Determine the system of differential equations and sketch the graph of molar flows and concentrations of compounds in the reaction medium over the (volume) of the reactor. Assume that there is no head loss. The reaction temperature is 300 K and is isothermal. Use Polymath.
CH2O+1/2O22CH2OHCOOHHCOOCH3+H2Ok1k2k3k4HCOOHHCOOCH3CO+H2OCH3OH+HCOOH k1=0,014exp[RE1(T13001)]L1/2mol1/2s1k2=0,007exp[RE2(T13001)]Lmol1s1k3=0,014exp[RE3(T13001)]s1k4=0,45exp[RE4(T13001)]Lmol1s1 E1=10,0kcalmol1E2=30,0kcalmol1E3=20,0kcalmol1E4=10,0kcalmol1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


