Question
It is intended to carry out on an industrial scale the reaction in liquid phase between the PFD and BTR compounds according to the following
It is intended to carry out on an industrial scale the reaction in liquid phase between the PFD and BTR compounds according to the following stoichiometry:
+ + 2
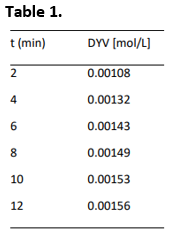
For their study in the laboratory, solutions were prepared (using 200 ml volumetric flasks) for the PFD compound and for the BTR compound using 4.3214 g and 0.1326 g respectively and the temperature was maintained at 345 K. The solutions were poured into a reactor of 1 L containing sufficient solvent so that the total volume of reaction was equal to that of the reactor. The technician followed the course of the reaction and obtained the data reported in Table 1. PFD and BTR have molecular weights of 34.12 and 154.98 g/mol respectively.
a) Calculate the necessary volume of a continuous stirred tank reactor (SCTR) to obtain 87.5% conversion of the reactants if a volumetric flow rate of 140 L/min is required.
b) Determine the volume for a plug flow reactor (RFP) operating under the same conditions as in part a).
c) Calculate the relationship between the volume of the reactors. Does your result make physical sense?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


