Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It is known that FD&C food dye Yellow # 5 (MM = 534.37 g/mol) has a Amax = 426 nm, while Blue #1 (MM
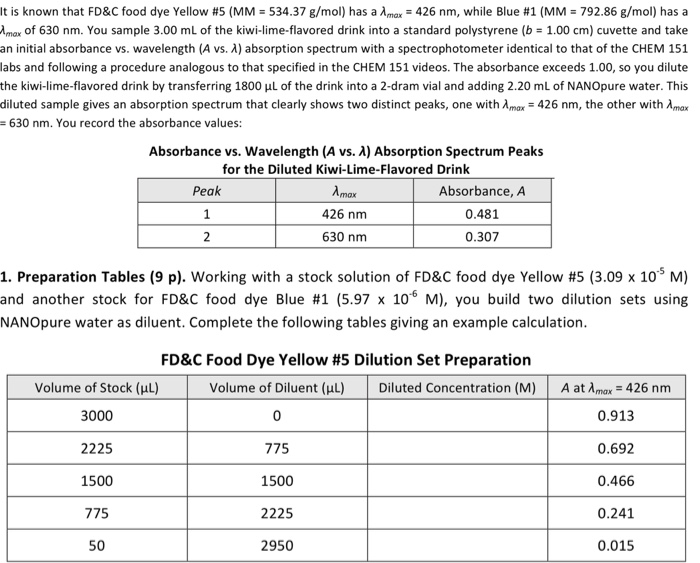
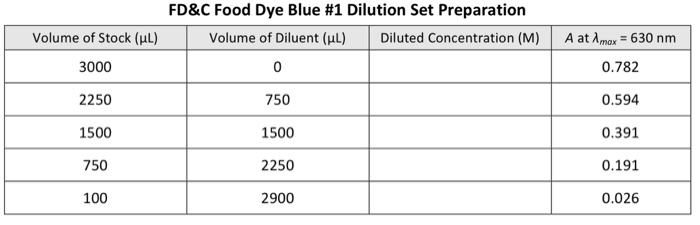


It is known that FD&C food dye Yellow # 5 (MM = 534.37 g/mol) has a Amax = 426 nm, while Blue #1 (MM = 792.86 g/mol) has a Amax of 630 nm. You sample 3.00 mL of the kiwi-lime-flavored drink into a standard polystyrene (b = 1.00 cm) cuvette and take an initial absorbance vs. wavelength (A vs. X) absorption spectrum with a spectrophotometer identical to that of the CHEM 151 labs and following a procedure analogous to that specified in the CHEM 151 videos. The absorbance exceeds 1.00, so you dilute the kiwi-lime-flavored drink by transferring 1800 L of the drink into a 2-dram vial and adding 2.20 mL of NANOpure water. This diluted sample gives an absorption spectrum that clearly shows two distinct peaks, one with Amax = 426 nm, the other with Amax 630 nm. You record the absorbance values: Absorbance vs. Wavelength (A vs. A) Absorption Spectrum Peaks for the Diluted Kiwi-Lime-Flavored Drink Peak 1 2 Volume of Stock (L) 3000 2225 1500 775 50 Amax 426 nm 630 nm 1. Preparation Tables (9 p). Working with a stock solution of FD&C food dye Yellow #5 (3.09 x 105 M) and another stock for FD&C food dye Blue #1 (5.97 x 10 M), you build two dilution sets using NANOpure water as diluent. Complete the following tables giving an example calculation. Absorbance, A 0.481 0.307 2950 FD&C Food Dye Yellow # 5 Dilution Set Preparation Volume of Diluent (l) 0 775 1500 2225 Diluted Concentration (M) A at Amax = 426 nm 0.913 0.692 0.466 0.241 0.015 Volume of Stock (l) 3000 2250 1500 750 100 FD&C Food Dye Blue # 1 Dilution Set Preparation Volume of Diluent (l) Diluted Concentration (M) 0 750 1500 2250 2900 A at Amax = 630 nm 0.782 0.594 0.391 0.191 0.026 2. Molar Absorptivity (8 p). Determine the molar absorptivity, , for the two dyes. Paste. Include the linear trendline equation(s) and R2 value(s). Clearly state the determined & for e 2. Molar Absorptivity (8 p). Determine the molar absorptivity, &, for the two dyes. Pastel Include the linear trendline equation(s) and R2 value(s). Clearly state the determined & for e
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


