Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It is required to design a separation process by distillation of the liquid mixture of solvents prior to a biochemical process. component 1: cyclohexanone component
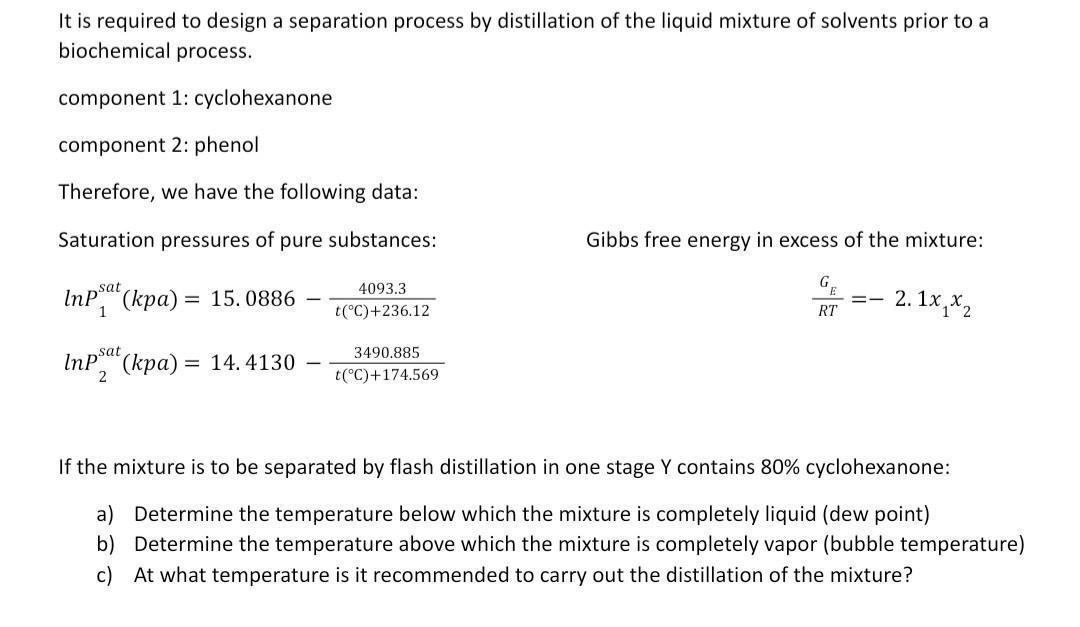
It is required to design a separation process by distillation of the liquid mixture of solvents prior to a biochemical process. component 1: cyclohexanone component 2: phenol Therefore, we have the following data: Saturation pressures of pure substances: Gibbs free energy in excess of the mixture: lnP1sat(kpa)=15.0886t(C)+236.124093.3RTGE=2.1x1x2ln2sat(kpa)=14.4130t(C)+174.5693490.885 If the mixture is to be separated by flash distillation in one stage Y contains 80% cyclohexanone: a) Determine the temperature below which the mixture is completely liquid (dew point) b) Determine the temperature above which the mixture is completely vapor (bubble temperature) c) At what temperature is it recommended to carry out the distillation of the mixture
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


