Question
(It's a physical chemistry problem) Consider a well-insulated piston-cylinder assembly shown above. On the 0.05 m2 piston rests two 5000-kg blocks. The initial temperature is
(It's a physical chemistry problem)
Consider a well-insulated piston-cylinder assembly shown above. On the 0.05 m2 piston rests two 5000-kg blocks. The initial temperature is 500.00 K. The ambient pressure is 5 bar. One mole of an ideal gas is contained in the cylinder. The gas is compressed in a process in which another 5000-kg block is added. The molar heat capacity at constant volume(Cv) can be taken to have a constant value of 5/2R and the gravitational constant is 9.81 ms-2
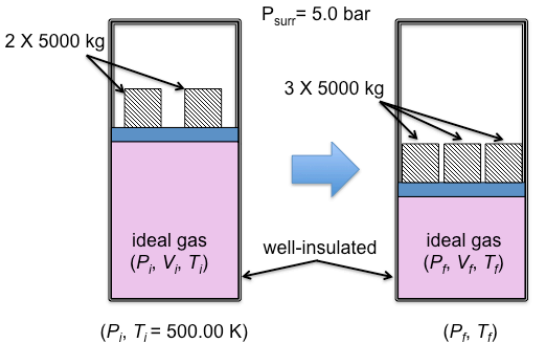
(2) Calculate Ssys and Ssurr (J mol-1 K-1). (Hint: 1) this is a case of an irreversible adiabatic compression, not a (reversible) isothermal compression; 2) you may be able to break down this process into 2 steps because the entropy (S) is a state function). All of your answers should have 3th decimal point (~.###))
(The answer in the book is (2) Ssurr = 0, Ssys = 0.354 J/molK)
Instead of simply mentioning (Ssurr = 0) in the correct answer above, please tell me how (Ssurr = 0) comes out.
Please write neatly so that I can read it easily and write the solution process so that the above answer comes out.
Psurr= 5.0 bar 2 X 5000 kg 3 X 5000 kg ideal gas (P; V;, T) well-insulated ideal gas (PF, V, T1) (Pi, T;= 500.00 K) (PF, T:)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


