Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It's finally summer and you decide to drive down to Disneyland to see Kristopher Barr's performance as Elsa on Ice. Unfortunately, your car breaks
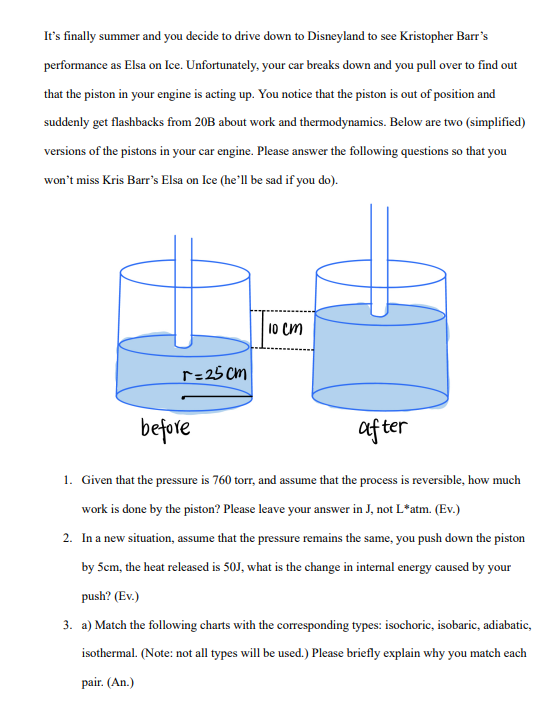
It's finally summer and you decide to drive down to Disneyland to see Kristopher Barr's performance as Elsa on Ice. Unfortunately, your car breaks down and you pull over to find out that the piston in your engine is acting up. You notice that the piston is out of position and suddenly get flashbacks from 20B about work and thermodynamics. Below are two (simplified) versions of the pistons in your car engine. Please answer the following questions so that you won't miss Kris Barr's Elsa on Ice (he'll be sad if you do). r=25cm 10 cm before after 1. Given that the pressure is 760 torr, and assume that the process is reversible, how much work is done by the piston? Please leave your answer in J, not L*atm. (Ev.) 2. In a new situation, assume that the pressure remains the same, you push down the piston by 5cm, the heat released is 50J, what is the change in internal energy caused by your push? (Ev.) 3. a) Match the following charts with the corresponding types: isochoric, isobaric, adiabatic, isothermal. (Note: not all types will be used.) Please briefly explain why you match each pair. (An.)
Step by Step Solution
★★★★★
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Well apply the formula for work performed in a reversible isothermal process to get the work performed by the piston ln WnRTln V f Where W stands for work completed n the quantity of gas not specified ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


