Answered step by step
Verified Expert Solution
Question
1 Approved Answer
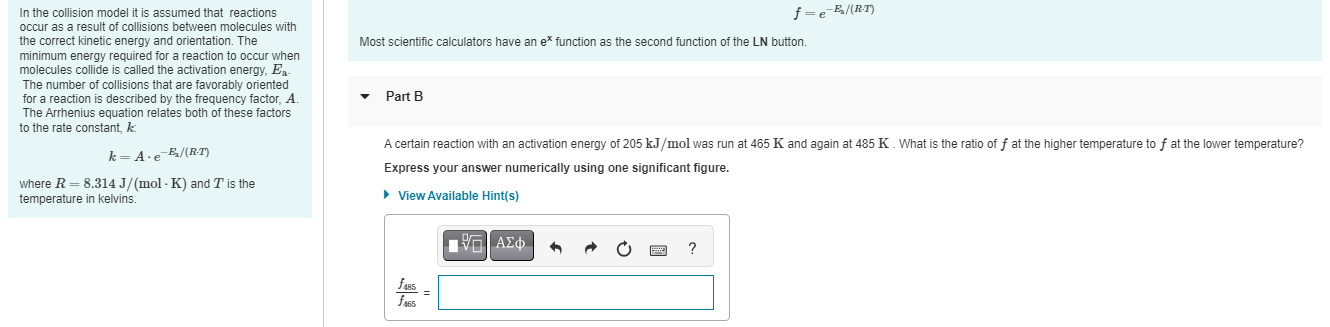
its urgent pleaseIn the collision model it is assumed that reactions occur as a result of collisions between molecules with the correct kinetic energy and
its urgent pleaseIn the collision model it is assumed that reactions
occur as a result of collisions between molecules with
the correct kinetic energy and orientation. The
minimum energy required for a reaction to occur when
molecules collide is called the activation energy,
The number of collisions that are favorably oriented
for a reaction is described by the frequency factor,
The Arrhenius equation relates both of these factors
to the rate constant, :
where and is the
temperature in kelvins.
Most scientific calculators have an function as the second function of the button.
Part B
A certain reaction with an activation energy of was run at and again at What is the ratio of at the higher temperature to at the lower temperature?
Express your answer numerically using one significant figure.
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


