Question: Which of the following molecules will have different values of standard molar enthalpy of formation, one calculated using bond energies and other calculated calorimetrically
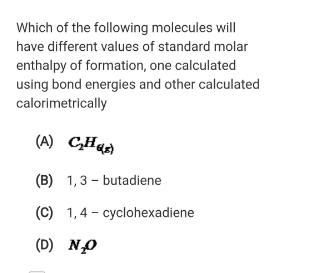
Which of the following molecules will have different values of standard molar enthalpy of formation, one calculated using bond energies and other calculated calorimetrically (A) CHaA (B) 1,3 - butadiene (C) 1,4 - cyclohexadiene (D) NO
Step by Step Solution
3.50 Rating (170 Votes )
There are 3 Steps involved in it
Option C is the answer Because here ... View full answer

Get step-by-step solutions from verified subject matter experts


