Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Iron (III) oxide (molar mass: 159.67 g/mol) reacts with carbon monoxide to produce iron and carbon dioxide. What is the percent yield for
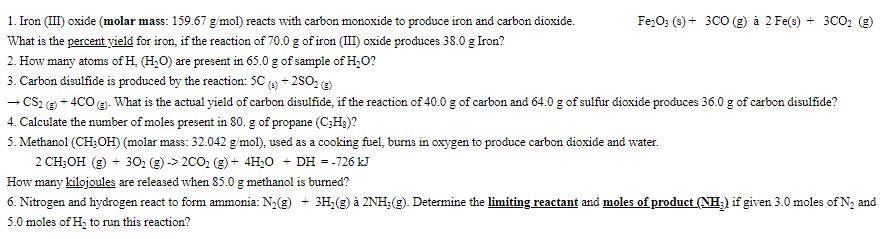
1. Iron (III) oxide (molar mass: 159.67 g/mol) reacts with carbon monoxide to produce iron and carbon dioxide. What is the percent yield for iron, if the reaction of 70.0 g of iron (III) oxide produces 38.0 g Iron? 2. How many atoms of H, (H2O) are present in 65.0 g of sample of HO? - 3. Carbon disulfide is produced by the reaction: 5C (s) + 2S02 (g) - Fe2O3 (s) + 3CO (g) 2 Fe(s) + 3CO2 (g) CS2 (g) +4CO(g). What is the actual yield of carbon disulfide, if the reaction of 40.0 g of carbon and 64.0 g of sulfur dioxide produces 36.0 g of carbon disulfide? 4. Calculate the number of moles present in 80. g of propane (CHg)? 5. Methanol (CH3OH) (molar mass: 32.042 g/mol), used as a cooking fuel, burns in oxygen to produce carbon dioxide and water. - 2 CH3OH (g) + 302 (g) -> 2002 (g) + 4HO - DH = -726 kJ How many kilojoules are released when 85.0 g methanol is burned? 6. Nitrogen and hydrogen react to form ammonia: N2(g) + 3H2(g) 2NH; (g). Determine the limiting reactant and moles of product (NH3) if given 3.0 moles of N and 5.0 moles of H to run this reaction?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


