Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.Calculate AG for the oxidation of one mole of methane, CH, in oxygen to form CO:(g) and H:0(1). AG =-817.617 2. How much heat
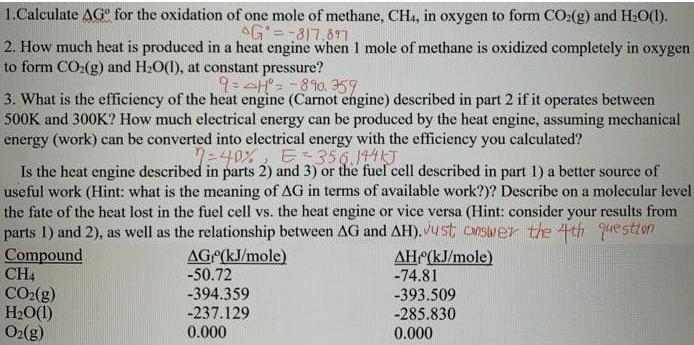
1.Calculate AG for the oxidation of one mole of methane, CH, in oxygen to form CO:(g) and H:0(1). AG =-817.617 2. How much heat is produced in a heat engine when I mole of methane is oxidized completely in oxygen to form CO:(g) and H2O(1), at constant pressure? 9:4H= -810. 359 3. What is the efficiency of the heat engine (Carnot engine) described in part 2 if it operates between 500K and 300K? How much electrical energy can be produced by the heat engine, assuming mechanical energy (work) can be converted into electrical energy with the efficiency you calculated? 7-40% E 356.141T Is the heat engine described in parts 2) and 3) or the fuel cell described in part 1) a better source of useful work (Hint: what is the meaning of AG in terms of available work?)? Describe on a molecular level the fate of the heat lost in the fuel cell vs. the heat engine or vice versa (Hint: consider your results from parts 1) and 2), as well as the relationship between AG and AH). Ust cswer the 4th question Compound CH4 CO2(g) H2O(1) O2(g) AH (kJ/mole) -74.81 -393.509 -285.830 0.000 AG (kJ/mole) -50.72 -394.359 -237.129 0.000
Step by Step Solution
★★★★★
3.48 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


