Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Just as a bond can be polar or nonpolar, we can also classify an entire molecule as polar or nonpolar. A molecule is said

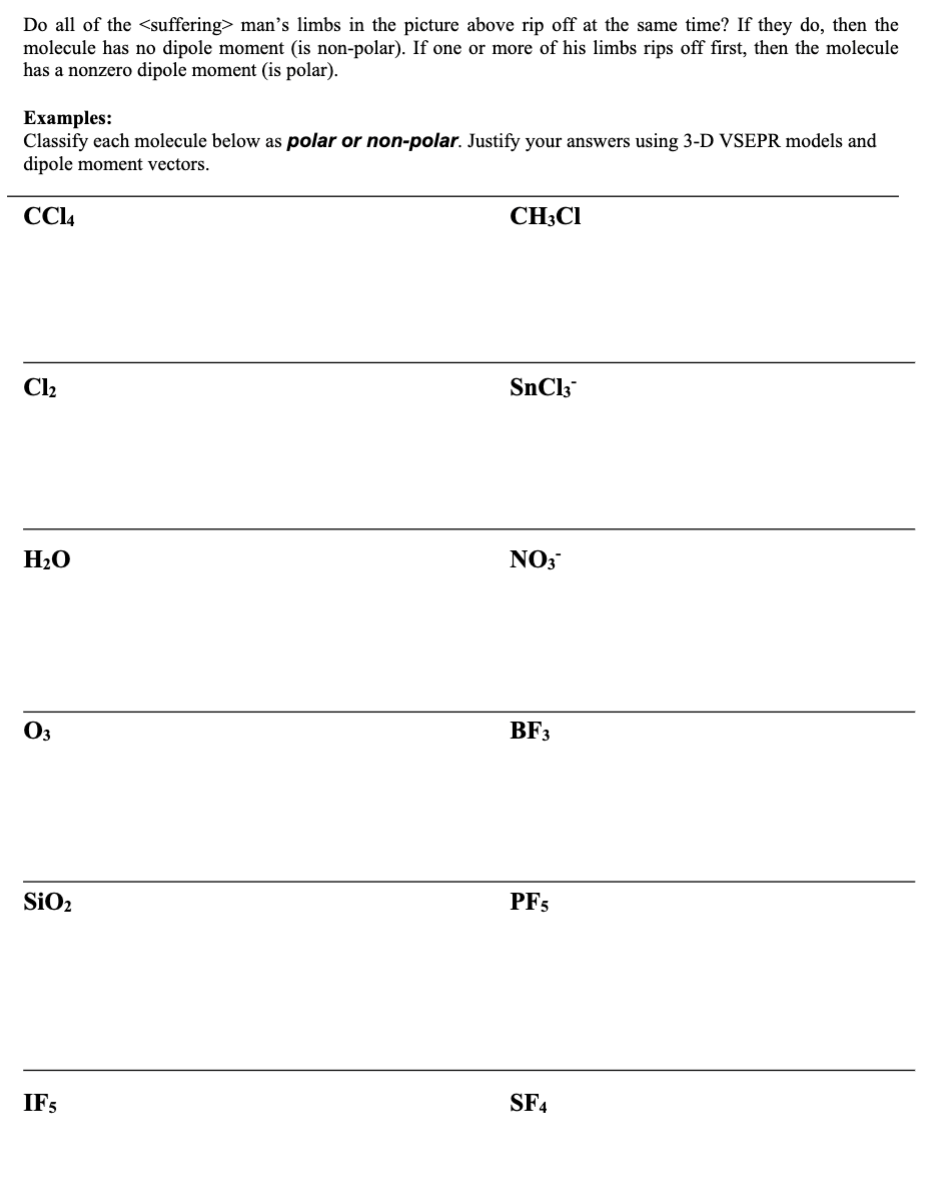
Just as a bond can be polar or nonpolar, we can also classify an entire molecule as polar or nonpolar. A molecule is said to be polar if the overall (net) effect of all the dipole moments in each bond do not cancel each other out. To determine polarity of a molecule, we first determine the molecular geometry and then draw an arrow pointing from the positive to negative side of each bond. The overall effect of each dipole is then considered. After you draw the molecular geometry, consider what would happen to the central atom if you tied a rope to each of the outside atoms and pulled. Or, if you're morbid (like the textbook I stole these pictures from) consider what would happen to the man below: trase He stays put... meaning the SO molecule is nonpolar. He moves. meaning the SOCI molecule is polar. Do all of the man's limbs in the picture above rip off at the same time? If they do, then the molecule has no dipole moment (is non-polar). If one or more of his limbs rips off first, then the molecule has a nonzero dipole moment (is polar). Examples: Classify each molecule below as polar or non-polar. Justify your answers using 3-D VSEPR models and dipole moment vectors. CC14 Cl HO 03 SiO IF5 CH3CI SnCl3 NO3 BF3 PF5 SF4
Step by Step Solution
★★★★★
3.52 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
answ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


