Question: Just aswer it, asap, thanks. Experiment #14: Pre-Lab Assignment Name The Six Test Tube Mystery Lab Instructor M Tu Th Lab Time Predict the products


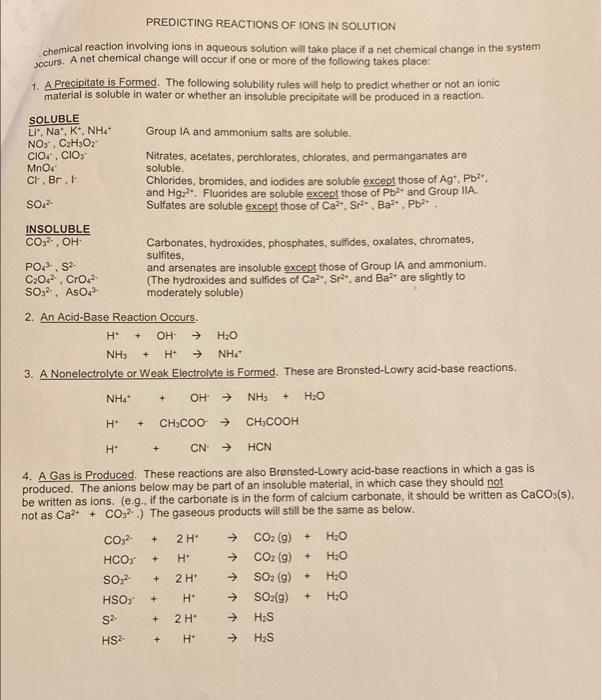
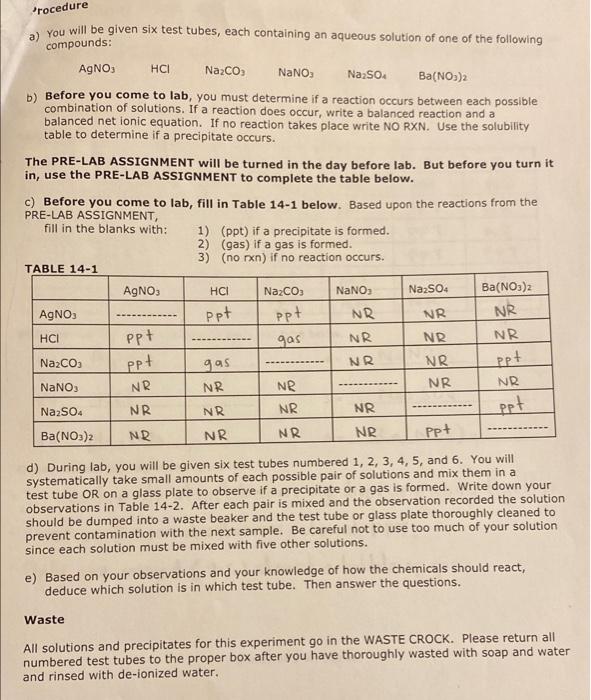
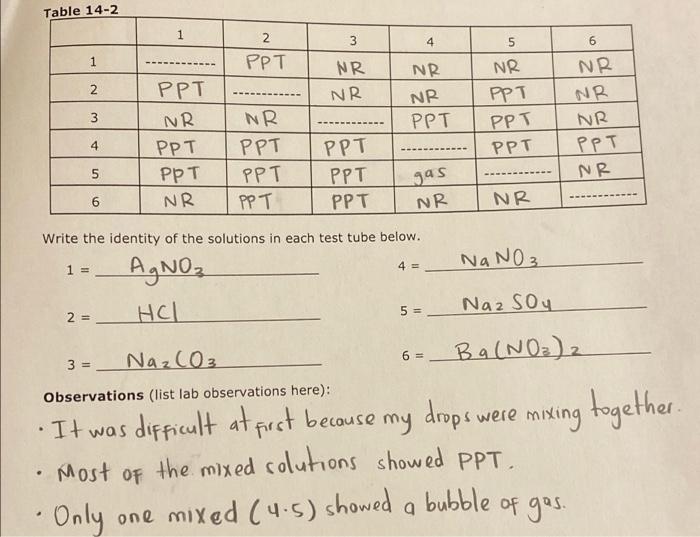

Experiment #14: Pre-Lab Assignment Name The Six Test Tube Mystery Lab Instructor M Tu Th Lab Time Predict the products of each reaction pair below and write a balanced equation. If a reaction will occur, write the net lonic equation as well. If no reaction occurs, write NO REACTION. You may wish to consult the rules for solubility on page 3 or in your lecture notes. (NIE = net ionic equation). 1) AgNO3(aq) HCl(aq) + NIE = (cancel the spectations ions A and N0,-) + > Ag Cles) + HNO3(aq) ANG 2) AgNO, (aq) + Na2CO3(aq) NIE = + NaNO, (aq) 3) AgNO3(aq) NIE = + 4) AgNO3(aq) Na2SO4 (aq) NIE = + 5) AgNO3(aq) Ba(NO3)2 NIE = > + Na2CO, (aq) 6) HCI (aq) NIE = 7) HCI (aq) + NaNO3(aq) NIE = HCI (aq) + Na2SO4 (aq) 1 NIE = 9) HCl(aq) Ba(NO3)2 (aq) NIE = 10) Na2CO3 (aq) + NaNO3(aq) NIE = 11) Na2CO3(aq) + Na2SO4 (aq) > NIE = 12) Na2CO3 (aq) + Ba(NO3)2 (aq) NIE = 13) NaNO3(aq) + Na2SO4 (aq) 1 NIE = 14) NaNO3(aq) + Ba(NO3)2 (aq) 1 NIE 15) Na2SO4 (aq) + Ba(NO3)2 (aq) NIE = PREDICTING REACTIONS OF IONS IN SOLUTION chemical reaction involving ions in aqueous solution will take place if a net chemical change in the system Securs. A net chemical change will occur if one or more of the following takes place: 1. A Precipitate is Formed. The following solubility rules will help to predict whether or not an ionic material is soluble in water or whether an insoluble precipitate will be produced in a reaction. SOLUBLE Li", Na, K, NH4 Group IA and ammonium salts are soluble. NO3. C.H.Oz CIOC. CIO Nitrates, acetates, perchlorates, chlorates, and permanganates are MnO4 soluble CH, Br. Chlorides, bromides, and iodides are soluble except those of Agt. Pb2, and Hgz?". Fluorides are soluble except those of Pb2 and Group IIA. Sulfates are soluble except those of Ca Sra. Baz. Pb? INSOLUBLE CO32-, OH Carbonates, hydroxides, phosphates, sulfides, oxalates, chromates, sulfites, PO3-. S. and arsenates are insoluble except those of Group IA and ammonium. C2042 CrO2 (The hydroxides and sulfides of Ca, Sr. and Ba?' are slightly to SO?AsO? moderately soluble) 2. An Acid-Base Reaction Occurs. H OH H2O NH + NH 3. A Nonelectrolyte or Weak Electrolyte is formed. These are Bronsted-Lowry acid-base reactions. SO + H NHA + OH H + CH3COO CH3COOH H CN HCN + 4. A Gas is Produced. These reactions are also Brnsted-Lowry acid-base reactions in which a gas is produced. The anions below may be part of an insoluble material, in which case they should not be written as ions. (e.g., if the carbonate is in the form of calcium carbonate, it should be written as CaCO3(s). not as Ca2+ + CO.2.) The gaseous products will still be the same as below. CO3 2 H CO2(g) + .0 HCO: H CO2 (9) + H2O SO, 2 H SO2 (9) + H20 HSO3 H SO2(g) H2O S2 2 H Has H H2S + + + + + HS2 + Procedure a) You will be given six test tubes, each containing an aqueous solution of one of the following compounds: AgNO3 HCI Nazco NaNO3 Na2SO4 Ba(NO3)2 b) Before you come to lab, you must determine if a reaction occurs between each possible combination of solutions. If a reaction does occur, write a balanced reaction and a balanced net ionic equation. If no reaction takes place write NO RXN. Use the solubility table to determine if a precipitate occurs. The PRE-LAB ASSIGNMENT will be turned in the day before lab. But before you turn it in, use the PRE-LAB ASSIGNMENT to complete the table below. Before you come to lab, fill in Table 14-1 below. Based upon the reactions from the PRE-LAB ASSIGNMENT, fill in the blanks with: 1) (ppt) if a precipitate is formed. 2) (gas) if a gas is formed. 3) (no rxn) if no reaction occurs. TABLE 14-1 AgNO3 HCI Na2CO3 NaNO3 Na2SO4 Ba(NO3)2 AgNO3 ppt NR HCI gas NR NR. NR. Na2CO3 ppt gas NR NR ppt NaNO3 NR NR. Na2SO4 NR NR NR NR Ba(NO3)2 NR NR NR NR Ppt ppt NR NR ppt NR NR NR d) During lab, you will be given six test tubes numbered 1, 2, 3, 4, 5, and 6. You will systematically take small amounts of each possible pair of solutions and mix them in a test tube OR on a glass plate to observe if a precipitate or a gas is formed. Write down your observations in Table 14-2. After each pair is mixed and the observation recorded the solution should be dumped into a waste beaker and the test tube or glass plate thoroughly cleaned to prevent contamination with the next sample. Be careful not to use too much of your solution since each solution must be mixed with five other solutions. e) Based on your observations and your knowledge of how the chemicals should react, deduce which solution is in which test tube. Then answer the questions. Waste All solutions and precipitates for this experiment go in the WASTE CROCK. Please return all numbered test tubes to the proper box after you have thoroughly wasted with soap and water and rinsed with de-ionized water. Table 14-2 1 3 4 5 2. PPT 1 NR 2 NR NR NR NR PPT PPT PPT 6 NR NR NR PPT NR 3 PPT NR PPT PPT NR PPT 4 NR PPT PPT PPT 5 PPT PPT PPT gas 6 NR NR. Write the identity of the solutions in each test tube below. NaNO3 1 = 4 = AgNO HCL 2 =_ 5 = Naz Soy NazC03 6 = 3 = Observations (list lab observations here): Ba(NO)2 It was difficult at first because my drops were mixing together the mixed solutions showed PPT. - Only one mixed (4.5) showed a bubble of gas. . Most of Questions 1) In this experiment, four different precipitates are formed. Write the chemical formulas and name each of these precipitates. A) B) C) D) 2) Which solution does not react with any of the other solutions? -) If sulfuric acid was added to sodium carbonate, which type of reaction would take place? Write a balanced reaction and a balanced net ionic equation for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


