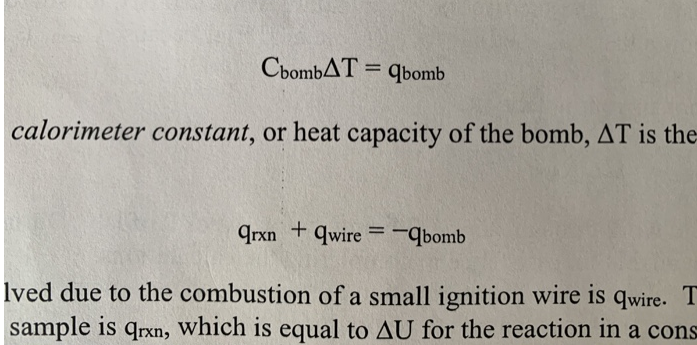
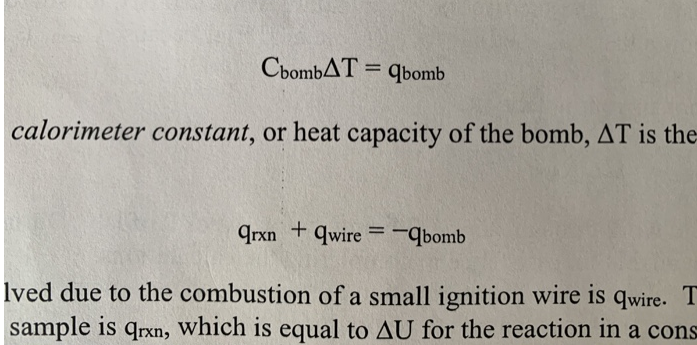





Just do the next question below: 
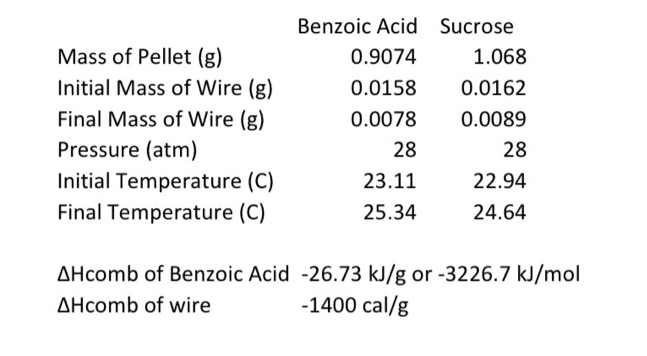
Hcomb of Benzoic Acid 26.73kJ/g or 3226.7kJ/mol Hcomb of wire 1400cal/g CbombT=qbomb calorimeter constant, or heat capacity of the bomb, T is the qrxn+qwire=qbomb lved due to the combustion of a small ignition wire is qwire.T sample is qrxn, which is equal to U for the reaction in a cons For benzoic acid Given data \& information Mass of pellet (g)=0.9074y Initial mas of wire (g)=0.0158g Final mass of wire (0)=0.0078y change in mass of uive (1) =0.002g Pressure (atm)=28atm Final Temperatere (Tf)=25.34C Initial Tempuatur (Ti)=23.11C Change in Tempeacture T=23.11C25.34C=2.23C Hkaib=26.73kJ/g Hcombofwire=1400cal/g (2) Literature value of Hcomb=26.73kJ/g Combustion reaction of benzoic acid C7H6O2(S)+215C2(g)214CO2(g)+3H2O(l) (Note: I am considering H2O is lipuid form) ng=(214215)=0.5mol change in mole of reaction of gas. Hcomb=Uconb+g Rat (defination of centhalpy) OR OR Ucomb=HcombngR. qrn=Ucomb=HcombngRT (Note: here T=2.98K, because H comb literature also) Rutting according required data from () qrn=Ucorb=26730gJ+1238.786JTocancelthis,bymeltiflyjinenmeightofbenzoicacidqrn=Ucomb=267g30J0.9074g+1238.786Jqren=Ucomb=24254.8J+1238.8Jqrin=Uccn=23016.05 (3.) qixn+qwire=qbamb We require qwire = ? gituen that \& Hcombofwire=1400cally 1cal=4.184J So Hcomb of wiore =14004.194gJ=5857.6gJ or [comb of wire =5857.6gJ] Note: wire is solid, 5o quome=Ucomb=Hcond]forwireHencequire=5857.6gJ To removing gunitby muelhiby by quire=5857.6gJ10.008g qwire=5857.6gJ1changeinmassofwire(g,0.008g[qwire=+46.8608J] (c) Now calcelating qucmb qren+qwire=qbouborqbonb=(qren+qwire)qbomb=(23016.0J+46.8608J)qboub=+22969.124J Now, Cbomb= ? we know SoCboub=TTqbomb Thus, Cbounb=2.23C22969.14J=10300.06J/C Hence, Cbomb=10300.06Fec (4) For sacrose Mars of Rellet of Sucrose =1.068g (4) For sacrose Mass of Rellet of sucrose =1.068g Mars of wire chanye( ()=0.0531 ChangeintemperatureofsucroseT2T1=(24.64=22.44)C Change intemp. dsucouse (T)=1.7C Nede Now using a gain equation 1qbomb=cbombT hore: Courb is same as bensoic acid, be cause we using same calorimiter bent. Io qbamb for sucrose is =10300.06Jx(1.7)toK Hence qbomb for sacrose is =10300.06J1.7=17510.11J qbomb=17510.11J for sucrase Now qren for sucrose A 80, quise. is ffohere q quire is same, beet mas different. so calculating H wire according same as equeation number (2) Hcombofwire1calfoHcombofofwire=2400callg=4.184J=14004.184gJ=5857.6gJ Note: wire is solid, 50 [fromineQN2[qwireUcomb=Hcont] for wire q1+41585.6gJ=2rn+42.76048J=17510.11Jqrn=17510.11J42.76048J=17552.761Jq12=17552.7615forsucrore (5.) SoUrn=17552.761J Combustion reaction for sucrose C12H22O11(s)+12O2(s)12CO2(g)+12H2O(l)ng=1212=0HenceHren=Usen+ORTHrxn=UrenHren=17552.7615fromcombastionreachon. 6. Based on your measured Hrxn for the combustion of sucrose, and literature values for Hf of O2,CO2 and H2O(l), calculate Hf for sucrose in kJ/mol. (Assume that STP conditions apply.)