Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Just need a reagent table for vial 1 shown, scheme is filled out thank you Prelab: Read through this procedure and skim the background Zubrick

Just need a reagent table for vial 1 shown, scheme is filled out thank you
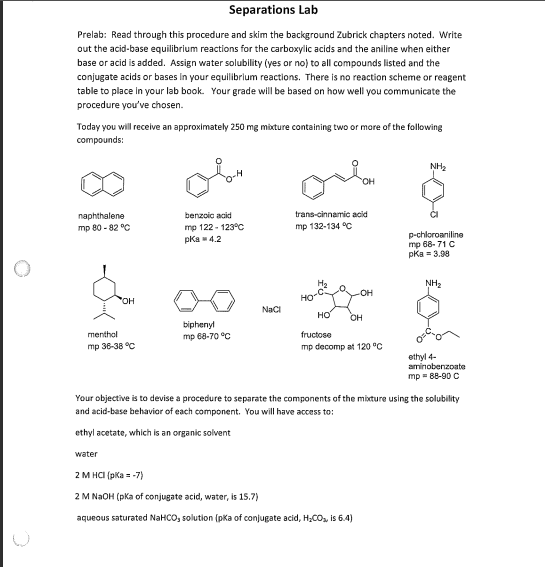
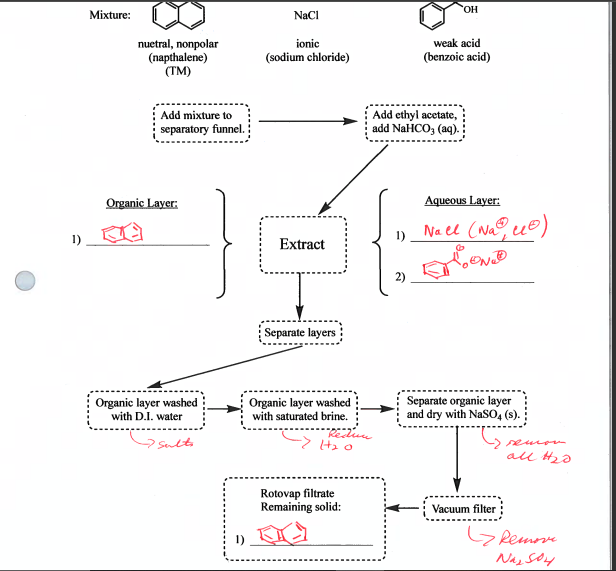
Prelab: Read through this procedure and skim the background Zubrick chapters noted. Write out the acid-base equilibrium reactions for the carboxylic acids and the aniline when elther base or acid is added. Assign water solubility (yes or no) to all compounds listed and the conjugate acids or bases In your equllibrium reactions. There is no reaction scheme or reagent table to place in your lab book. Your grade will be based on how well you communicate the procedure you've chosen. Today you will receive an approximately 250mg maxture containing two or more of the following compounds: Your objective is to devise a procedure to sepsrate the components of the mibture using the solubility and acid-base behavior of each component. You will have access to: ethyl acetate, which is an organic solvent water 2MHCl{pKa=7} 2MNaOH (pKa of conjugate acid, water, is 15.7) aqueous saturated NaHCO solution [pka of conjugate acid, H2CO2 is 6.4) saturated NaCl solution (often used to remove traces of water from a solution) sodium sulfate {Na2SO4], a drying agent You will need to devise a procedure based on extraction to separate the components. Here is a hint: for whichever liquid you choose to begin dissolving or washing your mixture, you will only need 510mL Details on extraction can be found in Zubrick in Chapter 15; Chapter 10 has some details on drying agents and Chapter 21 has information on rotary evaporation. It is recommended you devise a flow chart for your procedure. Talk through your plan with your lab partner. Show me your flow chart before you begin work. Your mixture will contain one component for which melting point data is given; the amount of this component is 150mg. Your measure of success with this lab will be how much of that 150mg you have successfully recovered (above 90mg is considered successful) and how pure it is as determined by melting point (within 5C of stated). If your component ends up in ethyl acetate, the solvent can be removed by rotary evaporation at the end to yield a solid product. Carboxylic acids, when reprotonated in water by the addition of acid, may precipitate out and will be able to be collected by vacuum filtration. Anlline, when deprotonated in water by the addition of base, may precipitate out and will be able to be collected by vacuum filtration. In your lab book, be sure to explain the procedure you chose: why did you do each part of the procedure? What were you hoping to separate out? Where did each of the components end up after each step? Be sure to address the amount you recovered of your target compound; you can express it as a percent recovery. Separations List: Vial \#1: 150mg naphthalene, 50mg NaCl, 50mg benzoic acid Mixture: NaCl nuetral, nonpolar (napthalene) ionic weak acid (TM) (benzoic acid) 1) Add ethyl acetate, add NaHCO3(aq) Aqueous Layer: 2) Separate layers
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


