Answered step by step
Verified Expert Solution
Question
1 Approved Answer
just need help with the 6 questions Post-lab Questions #01. (1 pt) List the three mobile phases (A, B & C) in increasing polarity (least

just need help with the 6 questions 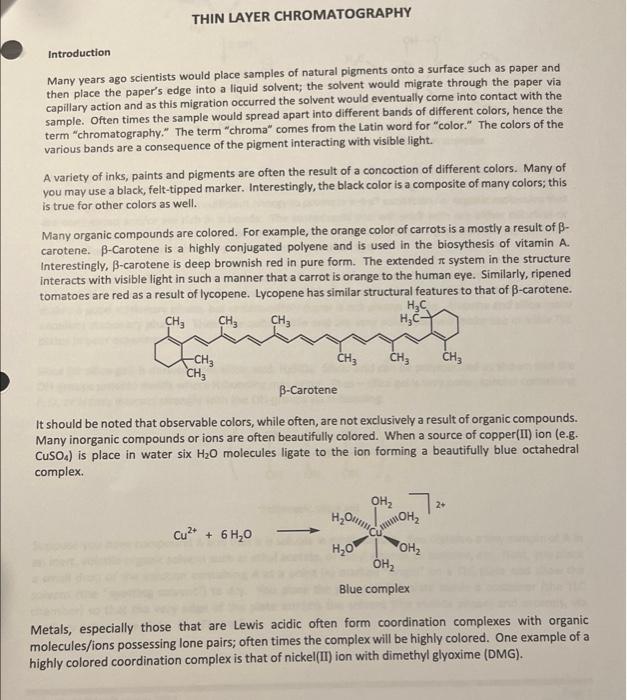
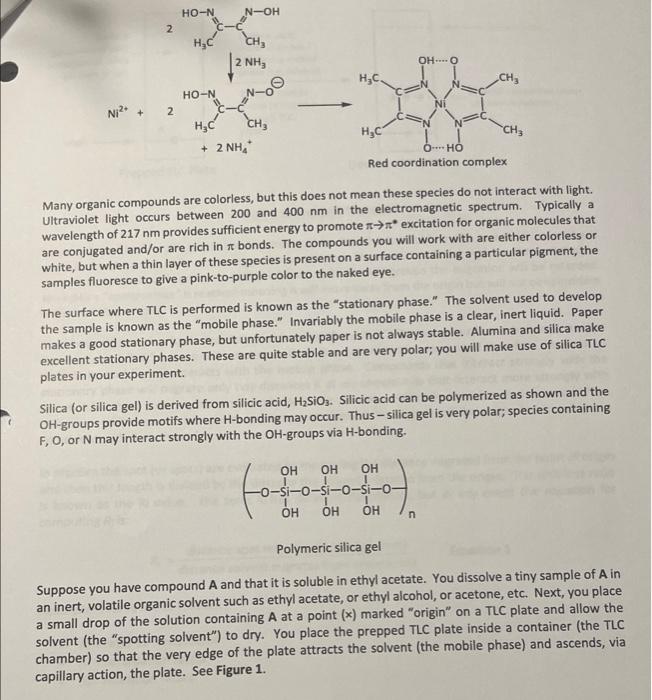

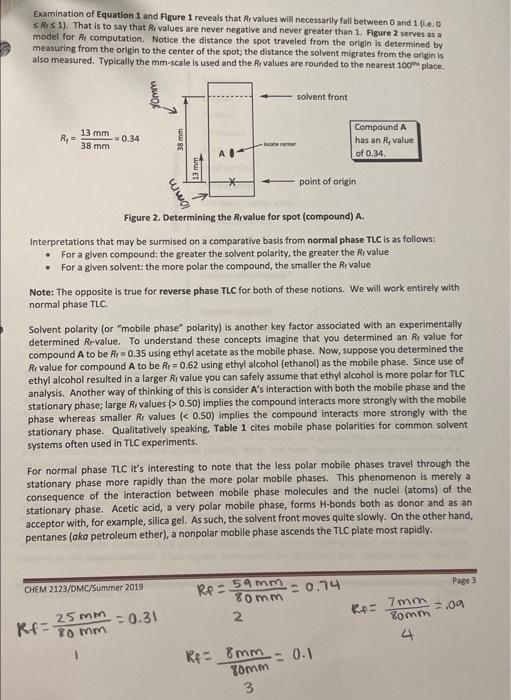


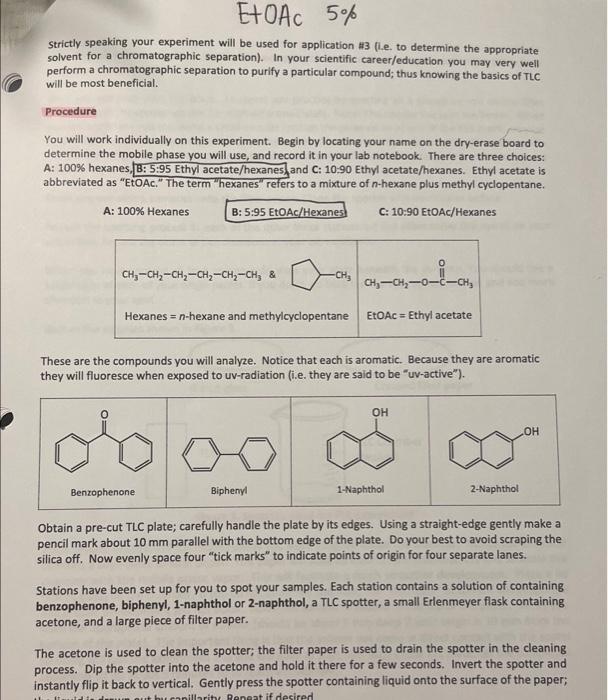

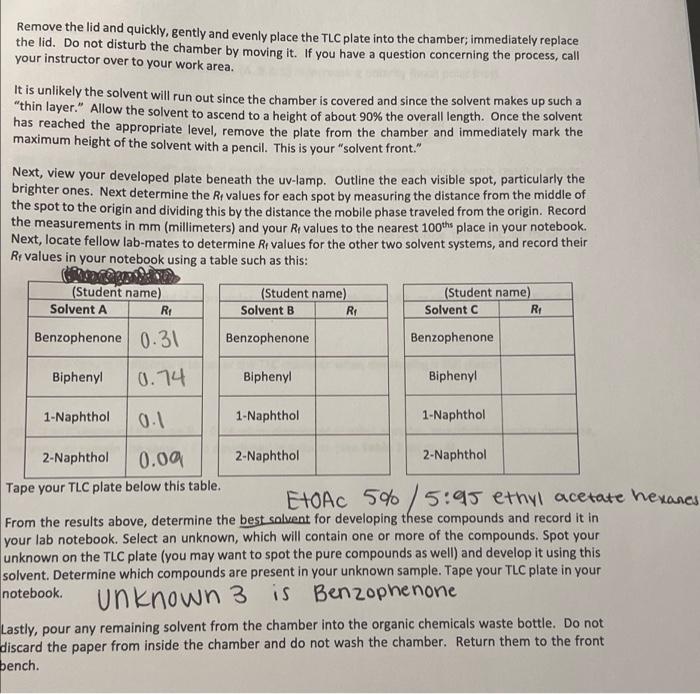
Post-lab Questions #01. (1 pt) List the three mobile phases (A, B & C) in increasing polarity (least polar first). least polar most polar #02. (2 pt) For the most part, do your overall results show that the more polar solvents result in greater Rr-values? Yes or no? Please elaborate briefly. #03. (2 pt) of the solvents investigated, which would be the best choice for a TLC of these four compounds? Justify your choice. #04.(1 pt) Which compound is more polar: Compound D with Rp = 0.43, or compound E with Rp = 0.25? #05. (2 pt) Why is it important to cover the TLC chamber when developing a TLC plate? #06. (1 pt)Compound Fhas an Rp = 0.34 when developed in ethyl acetate. Would the Rr-value decrease, increase, or remain the same if Fis developed in toluene (see Table 1 on pg.4). THIN LAYER CHROMATOGRAPHY Introduction Many years ago scientists would place samples of natural pigments onto a surface such as paper and then place the paper's edge into a liquid solvent; the solvent would migrate through the paper via capillary action and as this migration occurred the solvent would eventually come into contact with the sample. Often times the sample would spread apart into different bands of different colors, hence the term "chromatography." The term "chroma" comes from the Latin word for "color." The colors of the various bands are a consequence of the pigment interacting with visible light. A variety of inks, paints and pigments are often the result of a concoction of different colors. Many of you may use a black, felt-tipped marker. Interestingly, the black color is a composite of many colors; this is true for other colors as well. Many organic compounds are colored. For example, the orange color of carrots is a mostly a result of B- carotene. B-Carotene is a highly conjugated polyene and is used in the biosythesis of vitamin A Interestingly, B-carotene is deep brownish red in pure form. The extended to system in the structure Interacts with visible light in such a manner that a carrot is orange to the human eye. Similarly, ripened tomatoes are red as a result of lycopene. Lycopene has similar structural features to that of B-carotene. HC CHE CH3 CH3 HEC- CHE CH, CHE -CH3 CH B-Carotene It should be noted that observable colors, while often, are not exclusively a result of organic compounds. Many inorganic compounds or ions are often beautifully colored. When a source of copper(II)ion (e.g. CuSO4) is place in water six H2O molecules ligate to the ion forming a beautifully blue octahedral complex , 2+ Hround Cu2+ + 6H20 MANO OHZ OH, Blue complex Metals, especially those that are Lewis acidic often form coordination complexes with organic molecules/ions possessing lone pairs; often times the complex will be highly colored. One example of a highly colored coordination complex is that of nickel(II) ion with dimethyl glyoxime (DMG). HO-N N-OH 2 , CH3 2 NH3 OHO HC CHE HO-N Ni? 2 CH HC + 2 NH, HAC O HO Red coordination complex Many organic compounds are colorless, but this does not mean these species do not interact with light. Ultraviolet light occurs between 200 and 400 nm in the electromagnetic spectrum. Typically a wavelength of 217 nm provides sufficient energy to promote **** excitation for organic molecules that are conjugated and/or are rich in re bonds. The compounds you will work with are either colorless or white, but when a thin layer of these species is present on a surface containing a particular pigment, the samples fluoresce to give a pink-to-purple color to the naked eye. The surface where TLC is performed is known as the "stationary phase." The solvent used to develop the sample is known as the "mobile phase." Invariably the mobile phase is a clear, inert liquid. Paper makes a good stationary phase, but unfortunately paper is not always stable. Alumina and silica make excellent stationary phases. These are quite stable and are very polar, you will make use of silica TLC plates in your experiment. Silica (or silica gel) is derived from silicic acid, H2SIO3. Silicic acid can be polymerized as shown and the OH-groups provide motifs where H-bonding may occur. Thus-silica gel is very polar; species containing F, O, or N may interact strongly with the OH-groups via H-bonding. tor fofofo. OH OH OH -si-o-si-o-si-o- OH OH OH n Polymeric silica gel Suppose you have compound A and that it is soluble in ethyl acetate. You dissolve a tiny sample of A in an inert, volatile organic solvent such as ethyl acetate, or ethyl alcohol, or acetone, etc. Next, you place a small drop of the solution containing A at a point (*) marked "origin" on a TLC plate and allow the solvent (the "spotting solvent") to dry. You place the prepped TLC plate inside a container (the TLC chamber) so that the very edge of the plate attracts the solvent (the mobile phase) and ascends, via capillary action, the plate. See Figure 1. solvent front Surface (stationary phase) composed of silica gel (very polar) 1) Place plate Inside chamber containing mobile phase (i.e. solvent) 2) Allow mobile phase to migrate through TLC plate Normally visualized under ultraviolet radiation origin Prepped TLC Plate Developed TLC Plate Figure 1. Prepped TLC Plate Eventually the mobile phase will reach compound A. Recall A is soluble in the mobile phase and will want to move up the plate as the migration continues. However, recall the surface of the TLC plate possesses numerous OH-groups and if A interacts with the OH groups the migration will be retarded. Normally a "compromise" is reached meaning A will move away from the origin but will not be coincident with the solvent front The above scenario is a basic description of "normal phase TLC." There exist such a technique known as "reverse phase TLC," but we will not discuss this. The migration of a compound therefore depends on its polarity and how it interacts with the mobile phase. Compounds that interact strongly with the stationary phase will migrate only tiny distances if at all; compounds that interact more strongly with the mobile phase will migrate very easily with the solvent. Normally chemists desire to split-the- difference which means they prefer the compound/sample to migrate off the origin, but not migrate coincident with the solvent front. In practice the spot (or spots) are evaluated by measuring the distance migrated from the point of origin and then this length is divided by the distance the solvent migrated from the origin. This unit-less ratio is known as the Rr value. The term Rr has different descriptors, but in any event the equation for computing Reis: R, distance spot migrates from origin (mm) distance solvent migrates from origin (mm) Equation 1 Descriptors for Rare as follows: R = ratio-to-front factor R = retardation factor Ri= retention factor . Examination of Equation 1 and Figure 1 reveals that Revalues will necessarily fall between 0 and 1(e. O S R$ 1). That is to say that Rr values are never negative and never greater than 1. Figure 2 serves as a model for Recomputation Notice the distance the spot traveled from the origin is determined by measuring from the origin to the center of the spot; the distance the solvent migrates from the origin is also measured. Typically the mm-scale is used and the values are rounded to the nearest 100th place. Jom solvent front R,= 13 mm = 0.34 38 mm lacate care WWSE Www Compound A has an Rvalue of 0.34 point of origin lomm Figure 2. Determining the Rivalue for spot (compound) A. Interpretations that may be surmised on a comparative basis from normal phase TLC is as follows: For a given compound the greater the solvent polarity, the greater the R. value For a given solvent: the more polar the compound, the smaller the Rivalue Note: The opposite is true for reverse phase TLC for both of these notions. We will work entirely with normal phase TLC Solvent polarity or "mobile phase" polarity) is another key factor associated with an experimentally determined Rr-value. To understand these concepts imagine that you determined an Re value for compound A to be Re 0.35 using ethyl acetate as the mobile phase. Now, suppose you determined the Rr value for compound A to be Rp = 0.62 using ethyl alcohol (ethanol) as the mobile phase. Since use of ethyl alcohol resulted in a larger Rivalue you can safely assume that ethyl alcohol is more polar for TLC analysis. Another way of thinking of this is consider A's interaction with both the mobile phase and the stationary phase; large Ry values (> 0.50) implies the compound interacts more strongly with the mobile phase whereas smaller Ry values ( 








Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


