Question: Just Solve E 3D.2 Use the information on standard molar entropies in the Resource section to calculate the standard reaction entropy at 298K of (a)
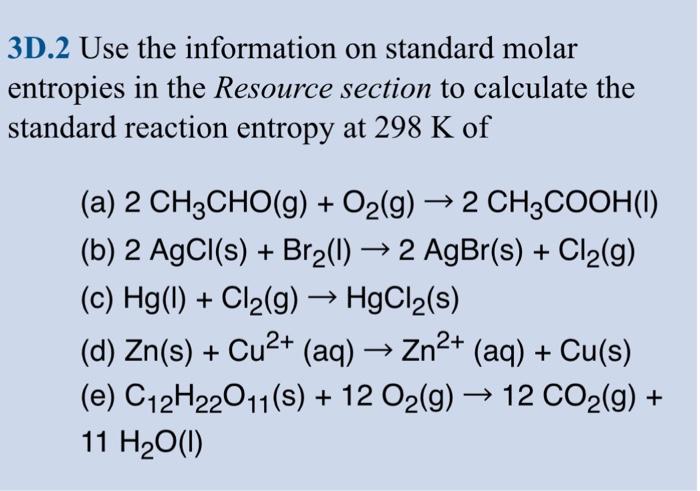
3D.2 Use the information on standard molar entropies in the Resource section to calculate the standard reaction entropy at 298K of (a) 2CH3CHO(g)+O2(g)2CH3COOH (l) (b) 2AgCl(s)+Br2(l)2AgBr(s)+Cl2(g) (c) Hg(l)+Cl2 (g) HgCl2 (s) (d) Zn(s)+Cu2+ (aq) Zn2+ (aq) +Cu(s) (e) C12H22O11(s)+12O2(g)12CO2(g)+ 11H2O(I) 3D.2 Use the information on standard molar entropies in the Resource section to calculate the standard reaction entropy at 298K of (a) 2CH3CHO(g)+O2(g)2CH3COOH (l) (b) 2AgCl(s)+Br2(l)2AgBr(s)+Cl2(g) (c) Hg(l)+Cl2 (g) HgCl2 (s) (d) Zn(s)+Cu2+ (aq) Zn2+ (aq) +Cu(s) (e) C12H22O11(s)+12O2(g)12CO2(g)+ 11H2O(I)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


