Answered step by step
Verified Expert Solution
Question
1 Approved Answer
just the last part! Reevaluate your calculations. Did you report your data to the correct number of significant figures? Volumesodiumcarbonate(mL)Molaritysodiumcarbonate(M)Volumecalciumchloride(mL)Molaritycalciumchloride(M)96.00.10100.00.10 Observations The mixture bubbled slightly
just the last part! 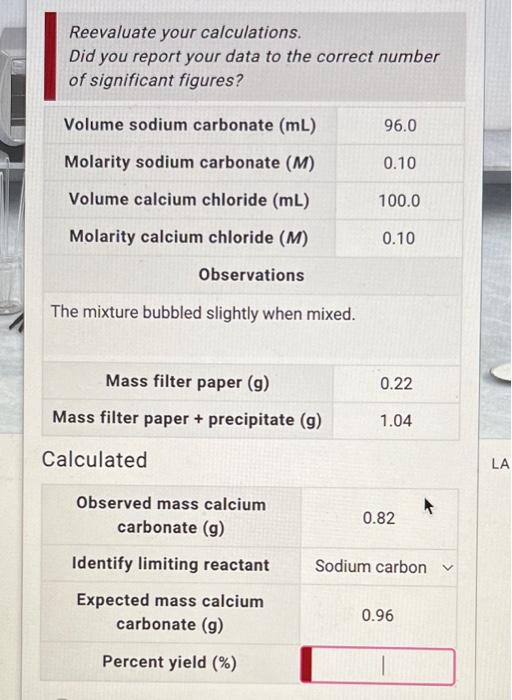
Reevaluate your calculations. Did you report your data to the correct number of significant figures? Volumesodiumcarbonate(mL)Molaritysodiumcarbonate(M)Volumecalciumchloride(mL)Molaritycalciumchloride(M)96.00.10100.00.10 Observations The mixture bubbled slightly when mixed. \begin{tabular}{c|c} \hline Mass filter paper (g) & 0.22 \\ \hline Mass filter paper + precipitate (g) & 1.04 \\ \hline \end{tabular} Calculated Observed mass calcium carbonate (g) 0.82 Identify limiting reactant Sodium carbon Expected mass calcium carbonate (g) 0.96 Percent yield (\%) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


