Answered step by step
Verified Expert Solution
Question
1 Approved Answer
kindly answer a & b Question 3 The Sutherland's viscosity law for gases has been derived from the kinetic theory by Sutherland using an idealized
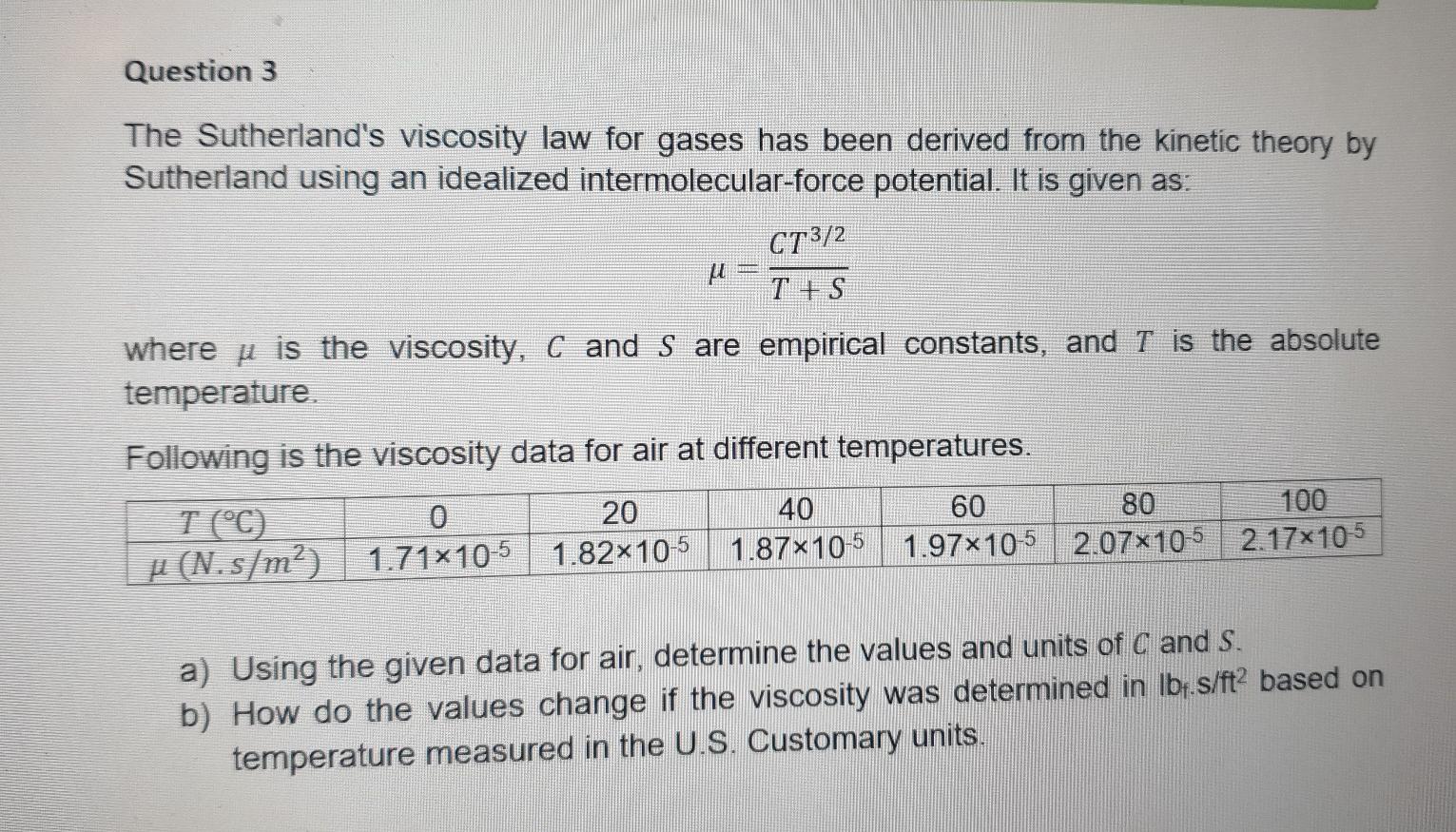
kindly answer a & b
Question 3 The Sutherland's viscosity law for gases has been derived from the kinetic theory by Sutherland using an idealized intermolecular-force potential. It is given as: CT3/2 U T + S where u is the viscosity, C and S are empirical constants, and T is the absolute temperature Following is the viscosity data for air at different temperatures. T (C) 20 40 60 80 100 u (N.s/m?) 1.71x10-5 1.82x10-5 1.87x10-5 1.97x10-5 2.07x10-5 2.17x105 a) Using the given data for air, determine the values and units of C and S. b) How do the values change if the viscosity was determined in lbf.s/ft2 based on temperature measured in the U.S. Customary unitsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


